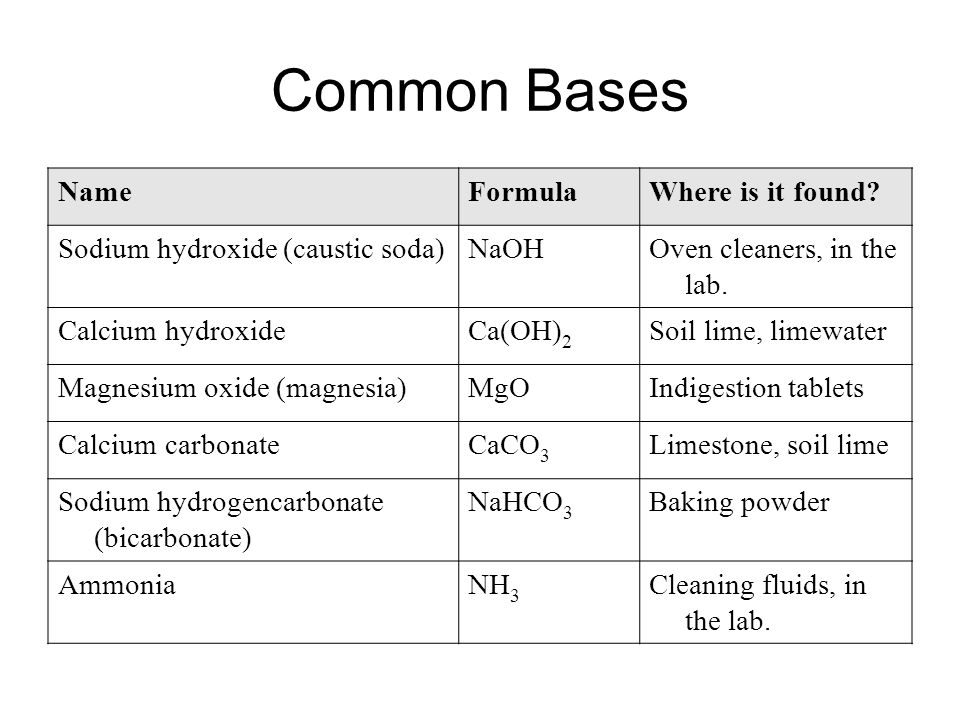
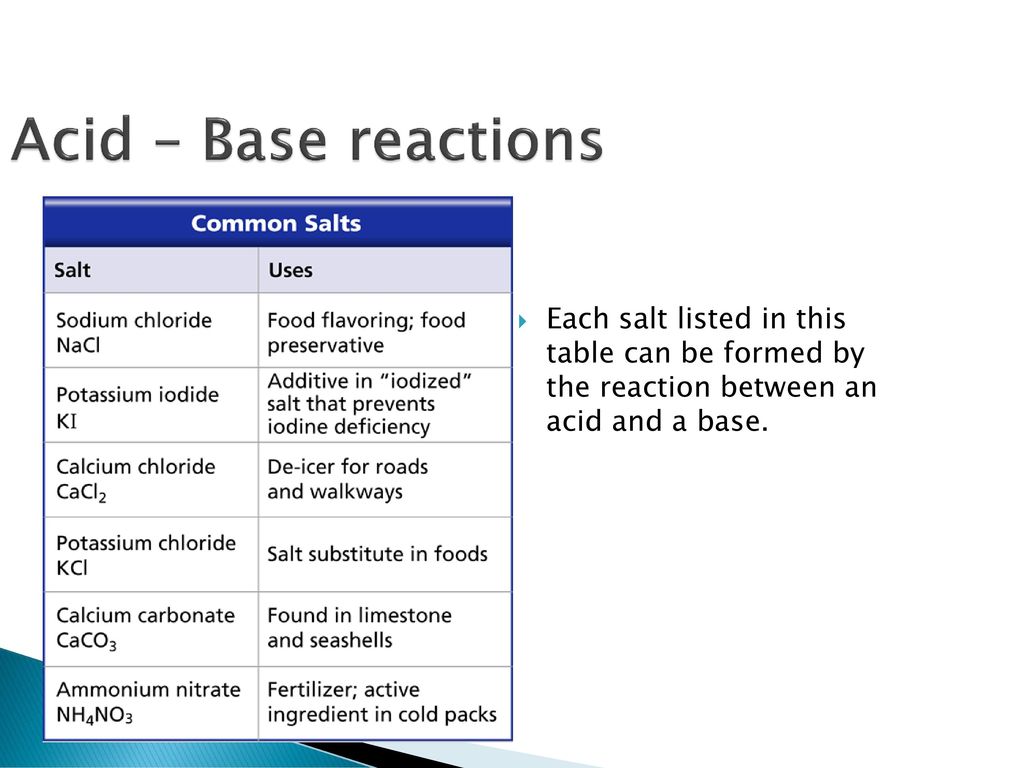
Acids, Bases and Salts Acids give up hydrogen ions (H+) in a water solution. Bases give up hydroxide ions (OH-) in a water solution. Mullis. - ppt video online download

Acid-base reaction of naproxen and calcium carbonate, containing the... | Download Scientific Diagram
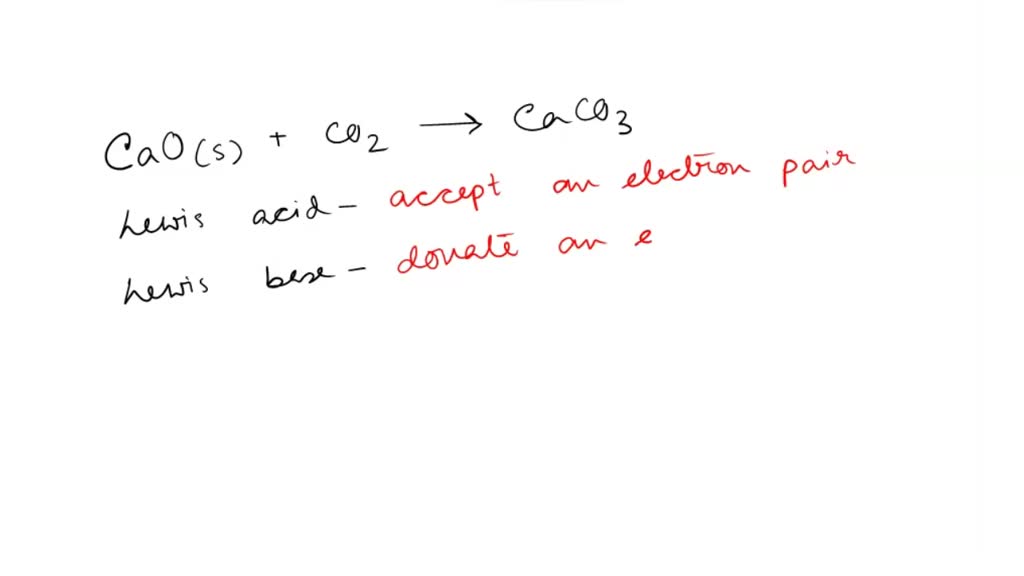
SOLVED: 'QUESTION 1 In the reaction: CaO(s) + C02(g) 5 CaCO3(s) 0A Ca2+acts as a Lewis acid and CO32- acts as a Lewis base 02-acts as a Lewis base and CO2 acts
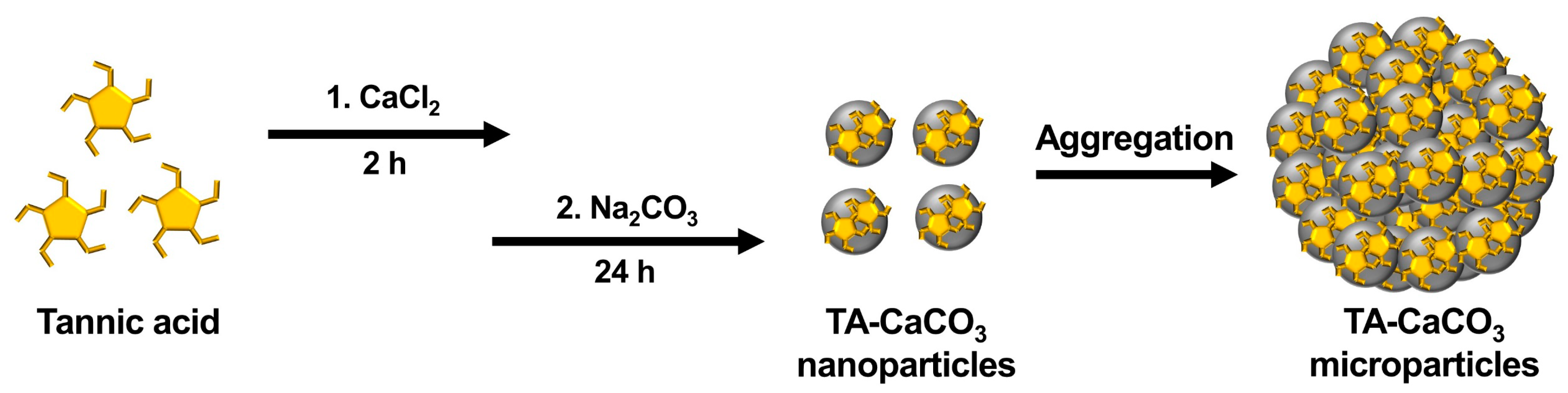
IJMS | Free Full-Text | Tannylated Calcium Carbonate Materials with Antacid, Anti-Inflammatory, and Antioxidant Effects
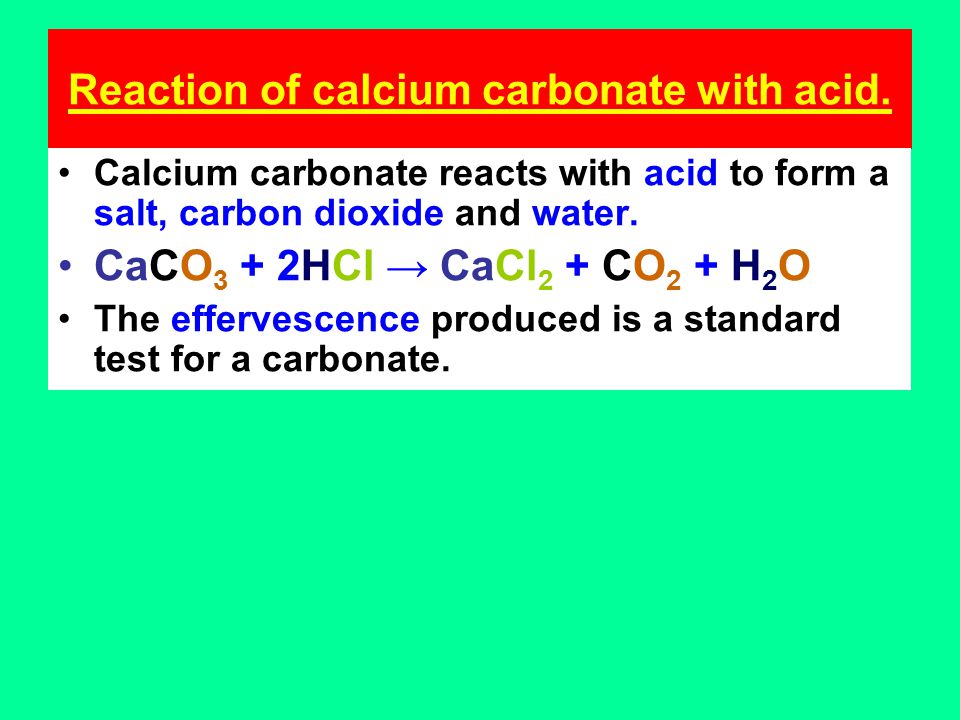
Everyday acid and base reactions. Calcium carbonate and rocks. Limestone is also largely composed of calcium carbonate. Bath Stone (Greater Oolite) is. - ppt download
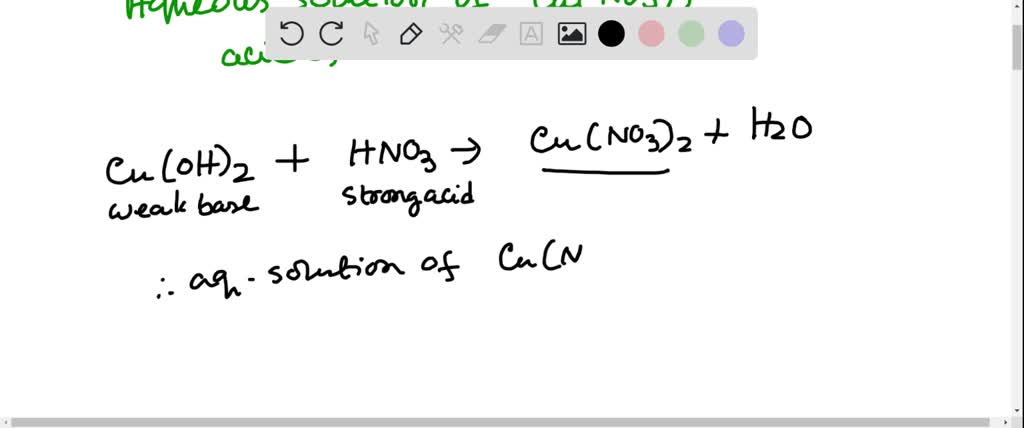
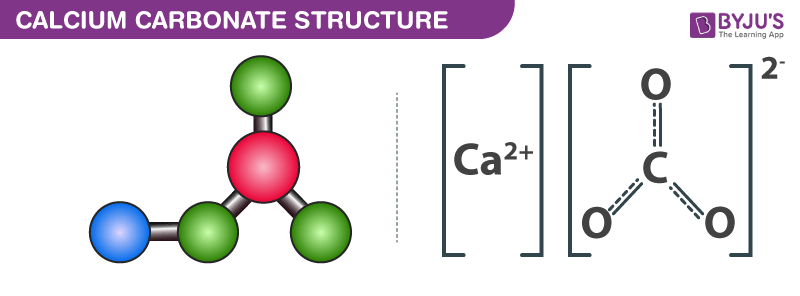
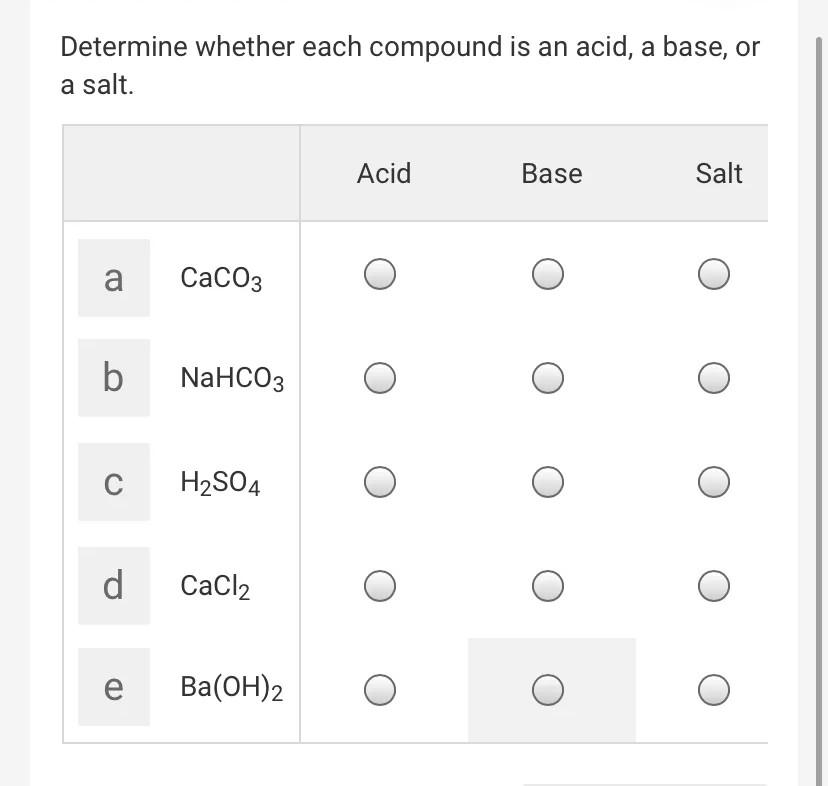
SOLVED: Solid nickel (III) nitrate, Ni(NO3)3, is dissolved in water. Is this solution acidic, basic, or neutral? Solid calcium carbonate, CaCO3, is dissolved in water. Is this solution acidic, basic, or neutral?

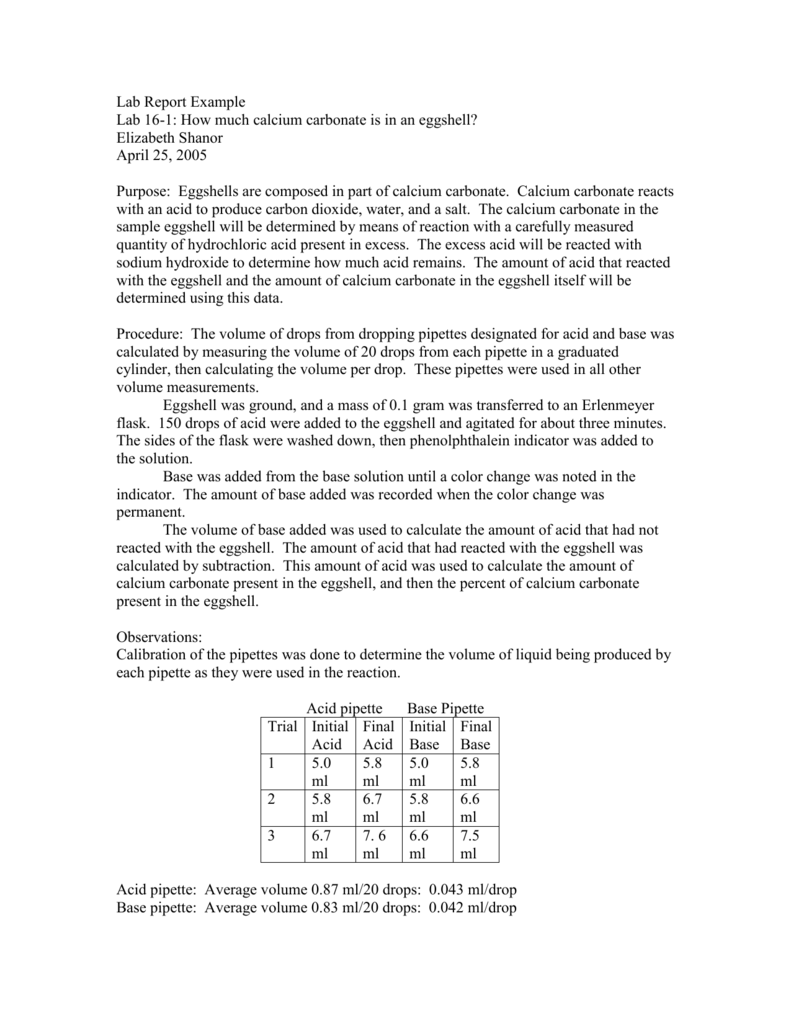
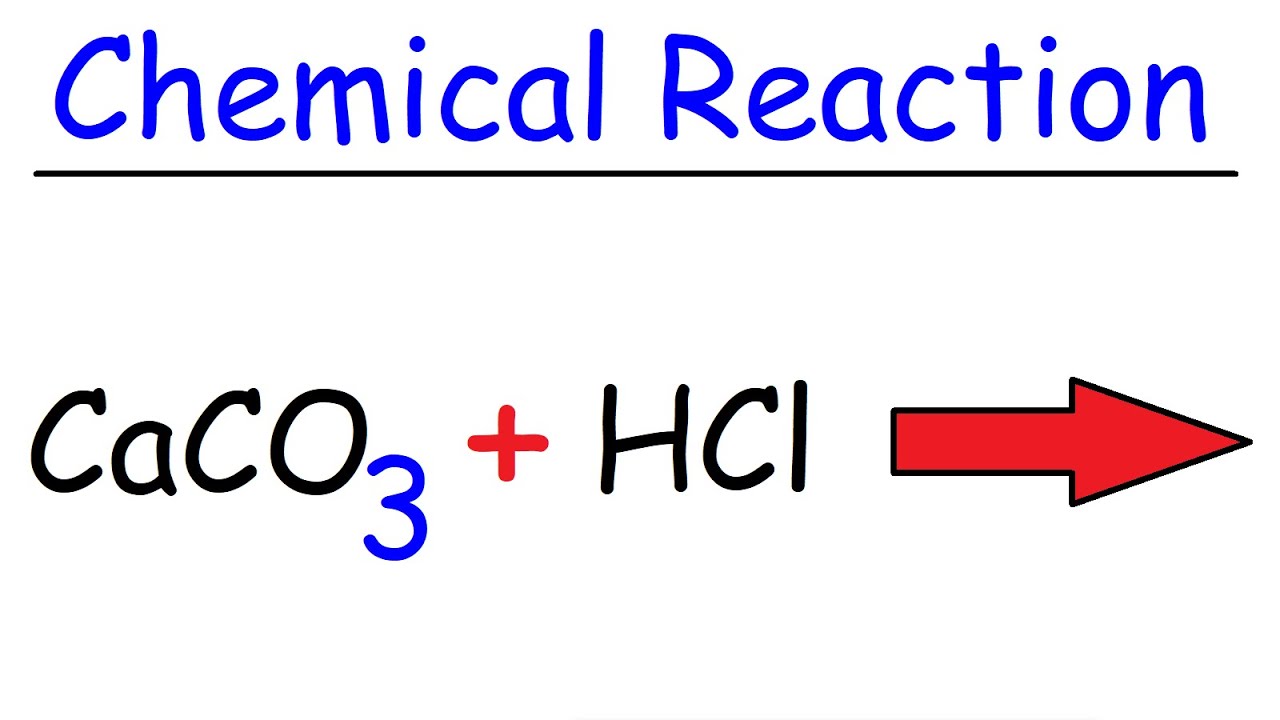
Calcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction, CaCO3 (s) + 2HCl (aq) →CaCl2 (aq) + CO2 (g) + H2O (l) .What mass of CaCO3
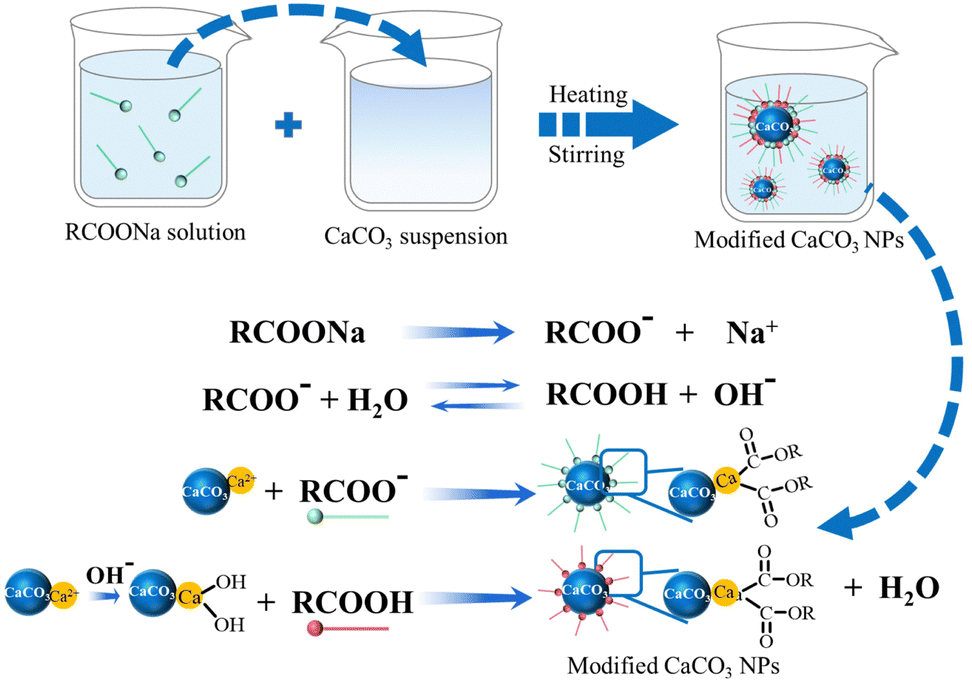

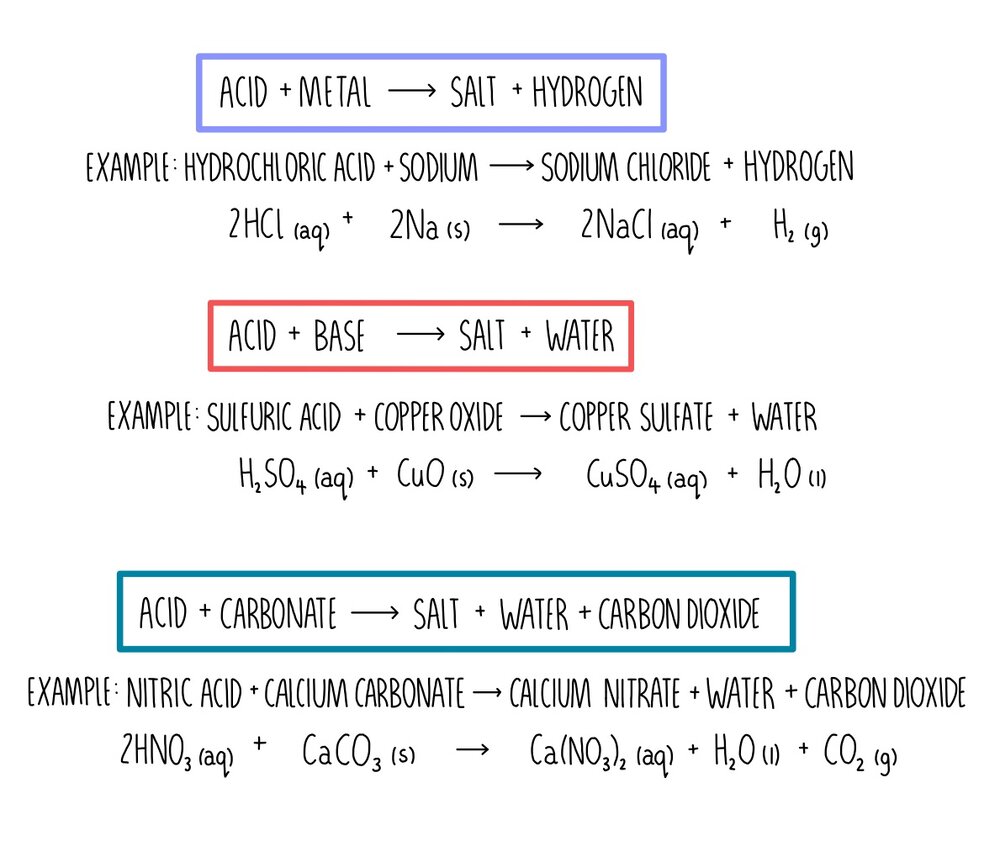
![MCQ] - Which correctly represents Parent acid and base of Calcium MCQ] - Which correctly represents Parent acid and base of Calcium](https://d1avenlh0i1xmr.cloudfront.net/0014c0c3-c848-4073-a814-6e71c0e2bf5e/reaction-to-form-calcium-carbonate---teachoo-01.jpg)