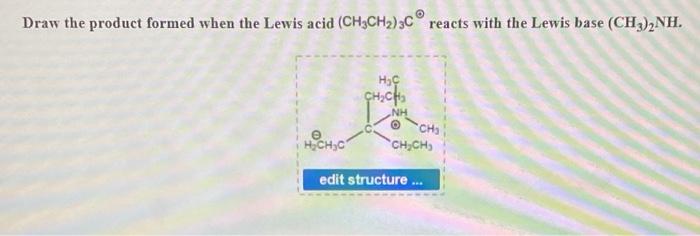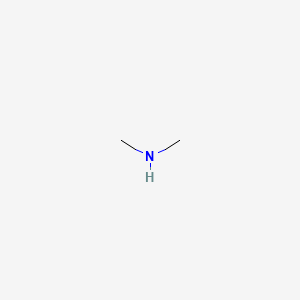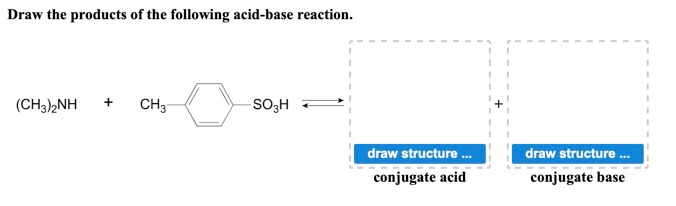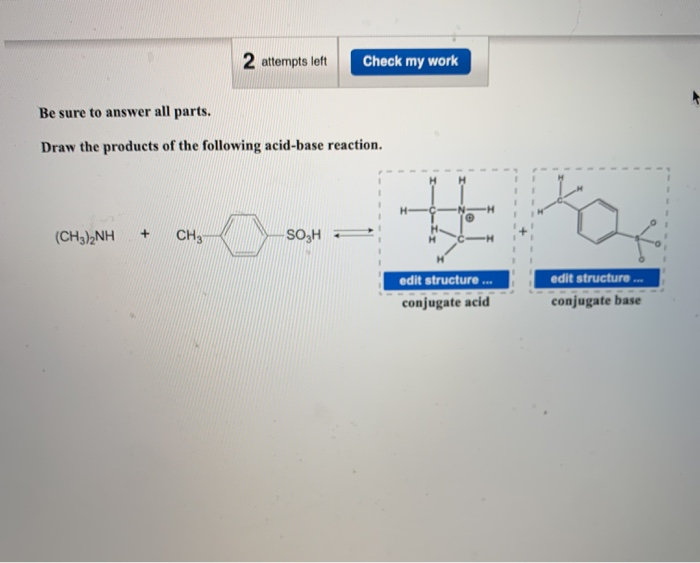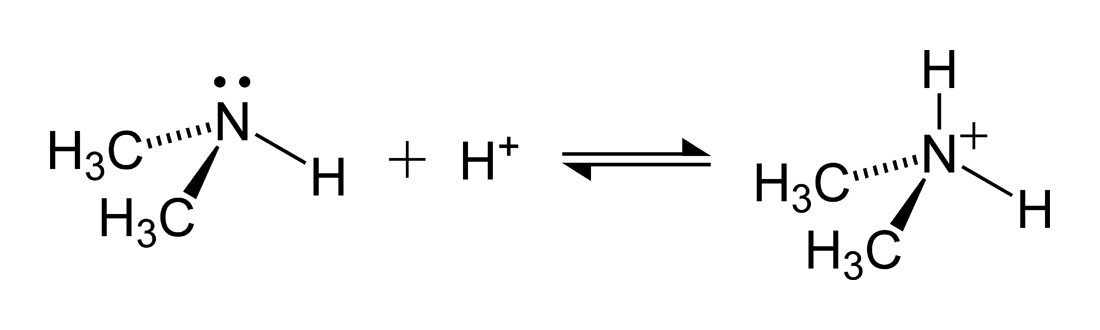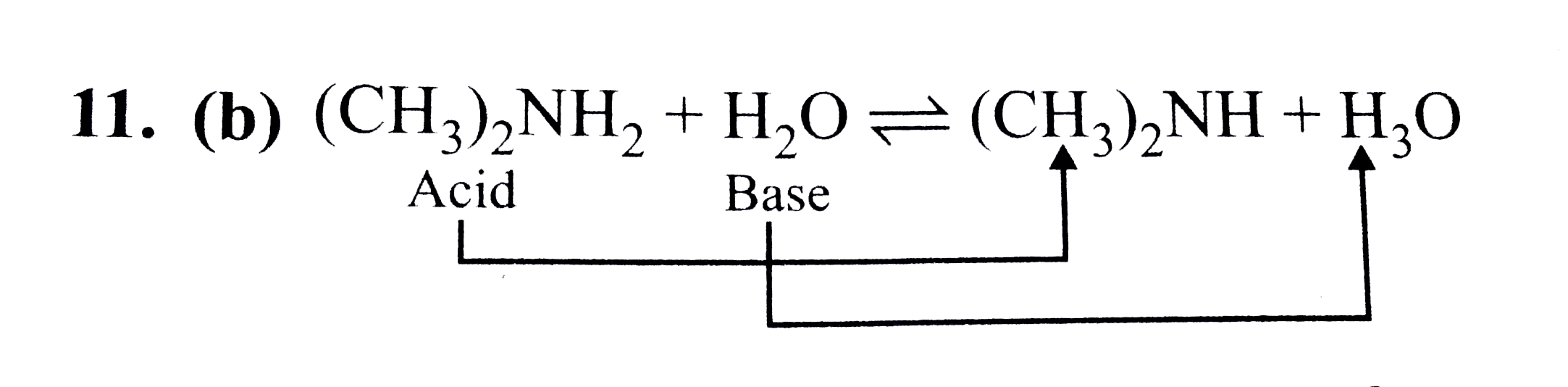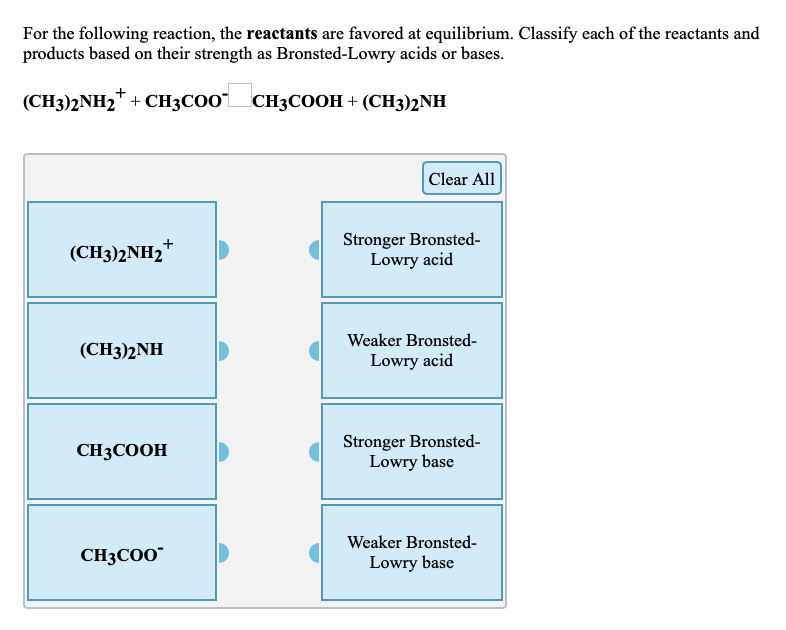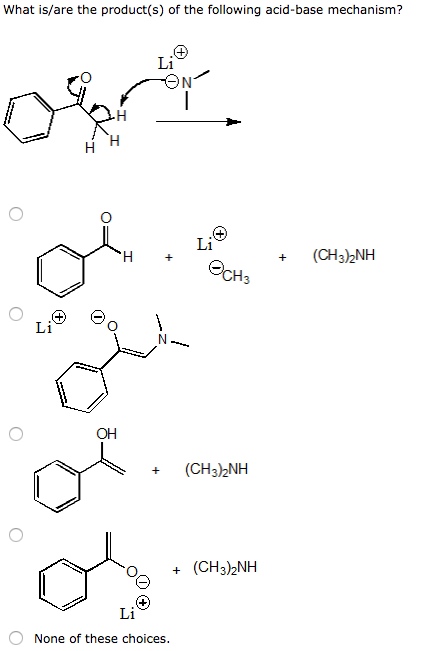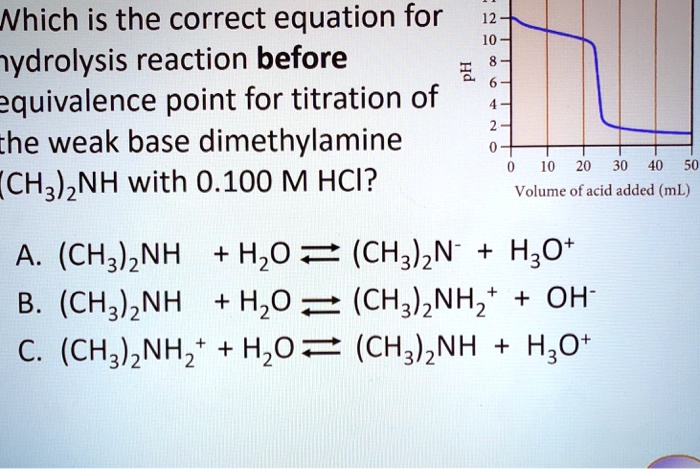
SOLVED: Nhich is the correct equation for ydrolysis reaction before 2 quivalence point for titration of he weak base dimethylamine (CHz)NH with 0.100 M HCI? Volume of acid added (mL) A (CH3)zNH

What is the order of basicity of the following compounds? CH3NH2, (CH3)2NH, (CH3)3N (in protic solvent)

Using the Brønsted-Lowry concept of acids and bases, which is the Brønsted-Lowry acid and base in the following reaction? (CH3)2NH(g)+BF3(g)→( CH3)2NHBF3(s) | Socratic

SOLVED: 1.) A 21.4 mL sample of 0.271 M dimethylamine, (CH3)2NH, is titrated with 0.372 M nitric acid. After adding 23.5 mL of nitric acid, the pH is . 2.) A 29.4