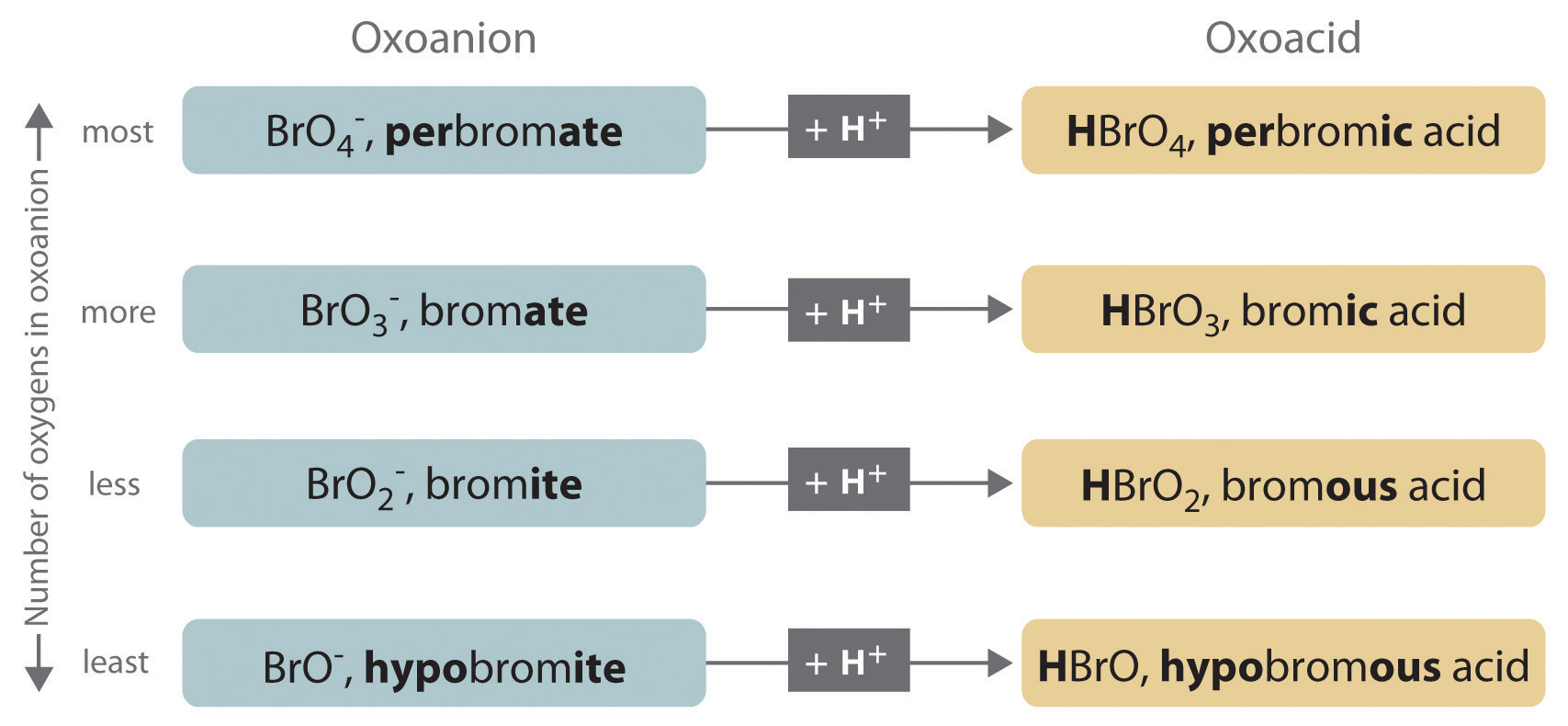
Lesson 5: Determining the strength of an acid or base - aeterniti | Chemistry | Vingle, Interest Network

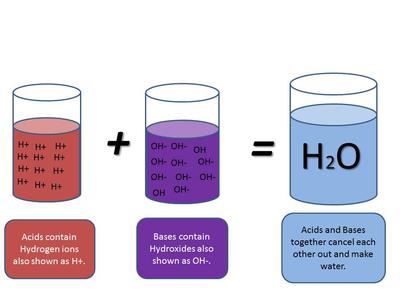
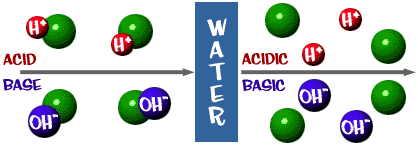
Acids, Bases and pH. 1. Acids Any compound that GIVES OFF H+ ions in solution Any compound that GIVES OFF H+ ions in solution Ex. HCl H+ and Cl- Ex. HCl. -

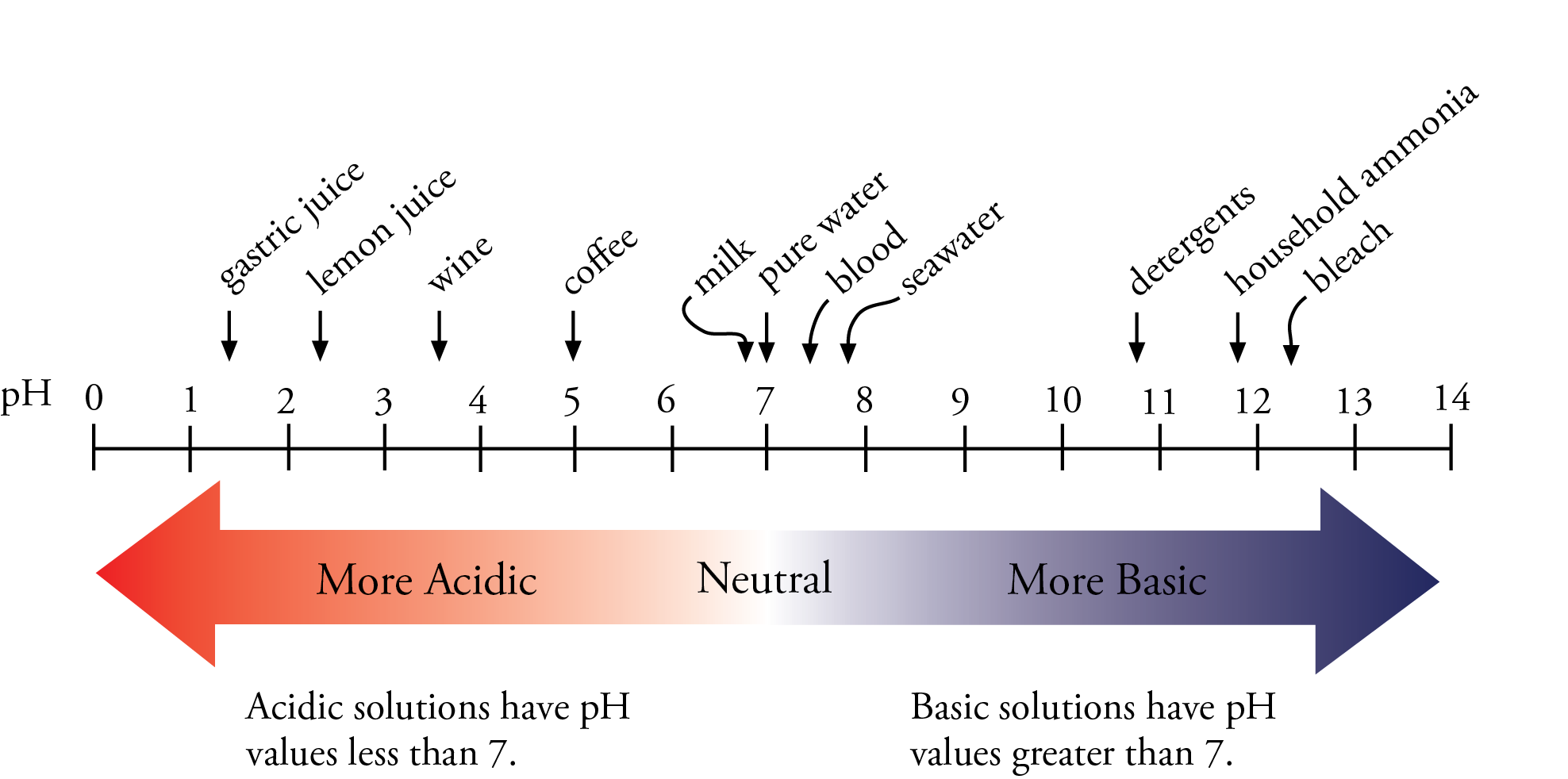
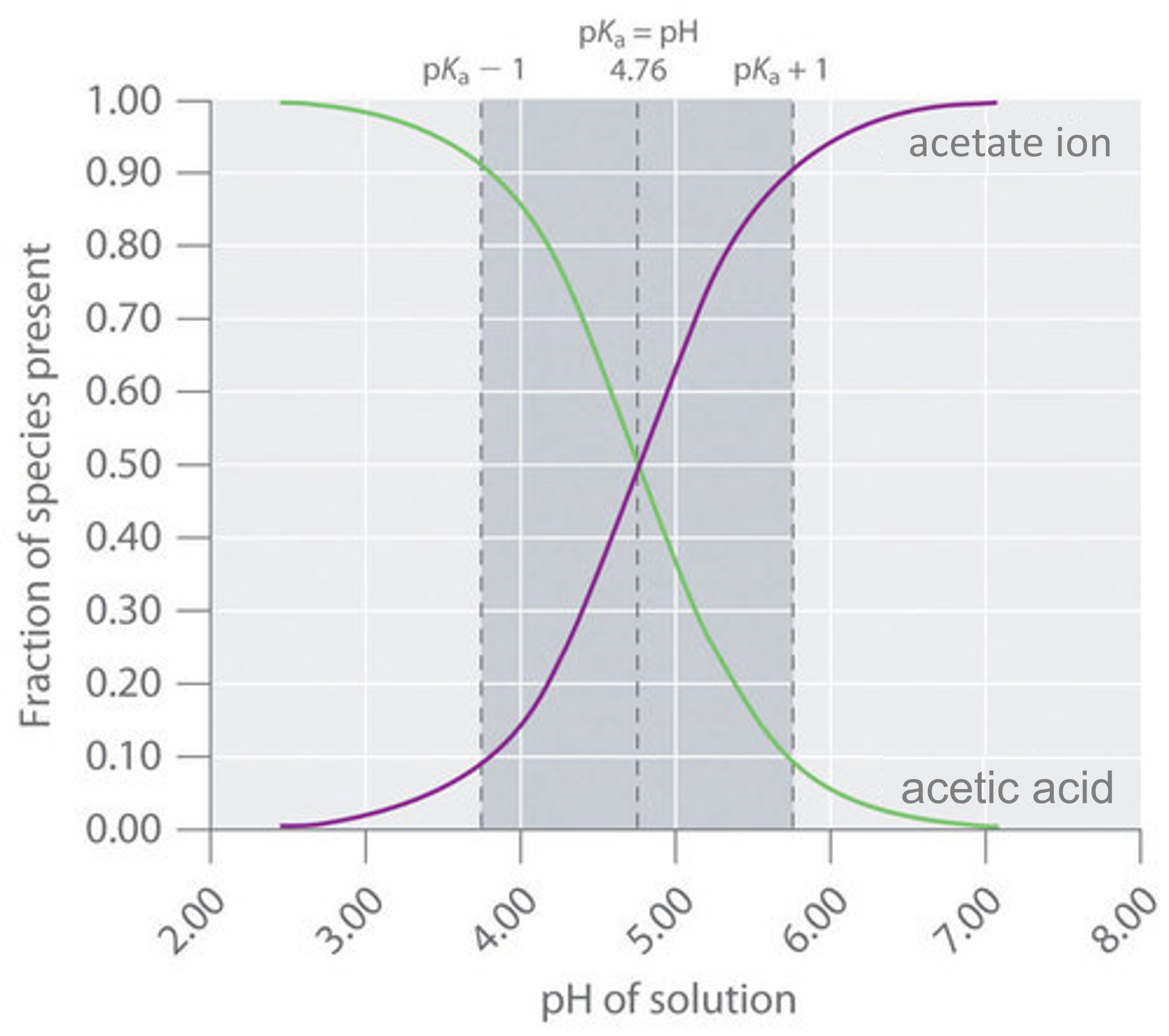
Determining PH of a Solution | Acidic, Basic & Neutral Solutions - Video & Lesson Transcript | Study.com
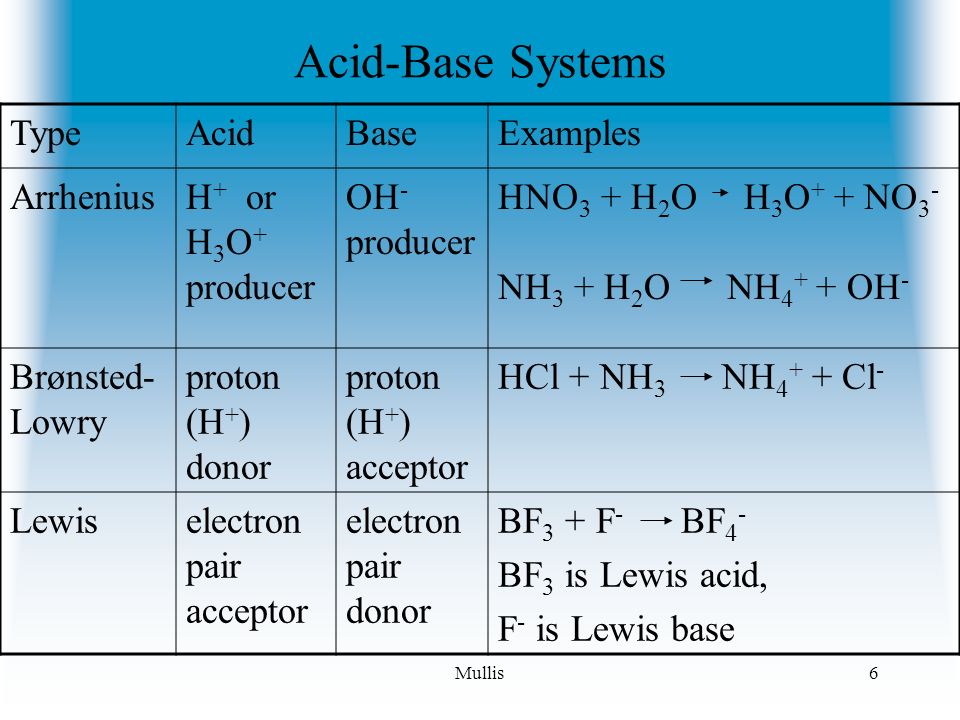
Acids, Bases and Salts Acids give up hydrogen ions (H+) in a water solution. Bases give up hydroxide ions (OH-) in a water solution. Mullis. - ppt video online download


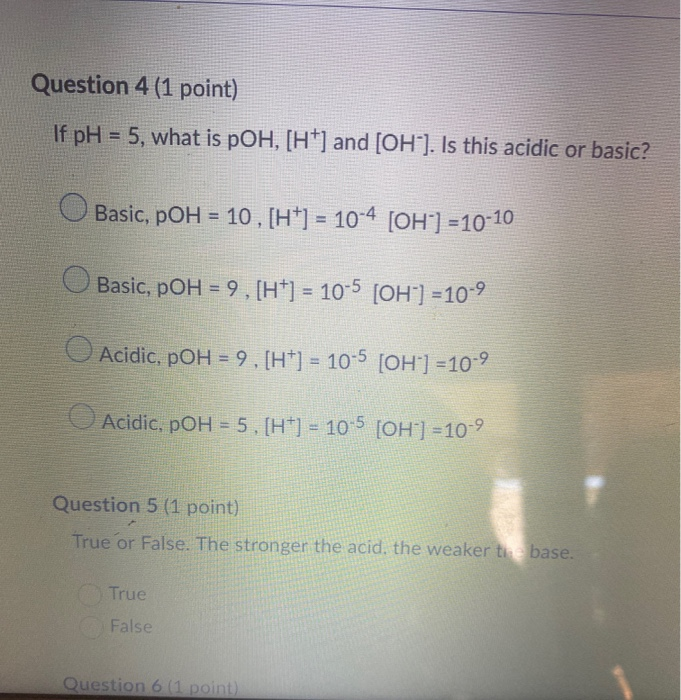
![Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and Bases - Practice - YouTube Calculating pH, pOH, [H+], [H3O+], [OH-] of Acids and Bases - Practice - YouTube](https://i.ytimg.com/vi/UiK37I159fc/maxresdefault.jpg)
.PNG)