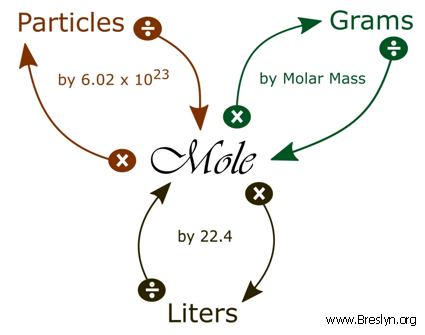
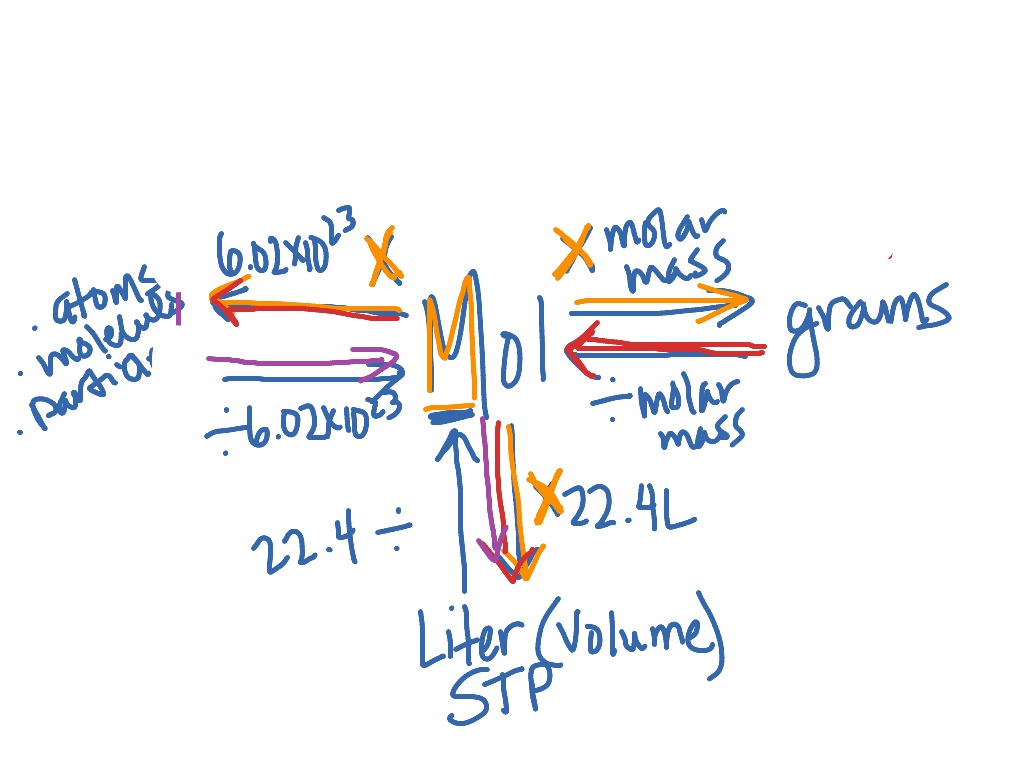
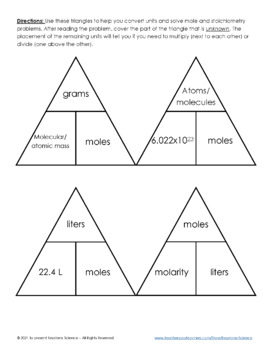
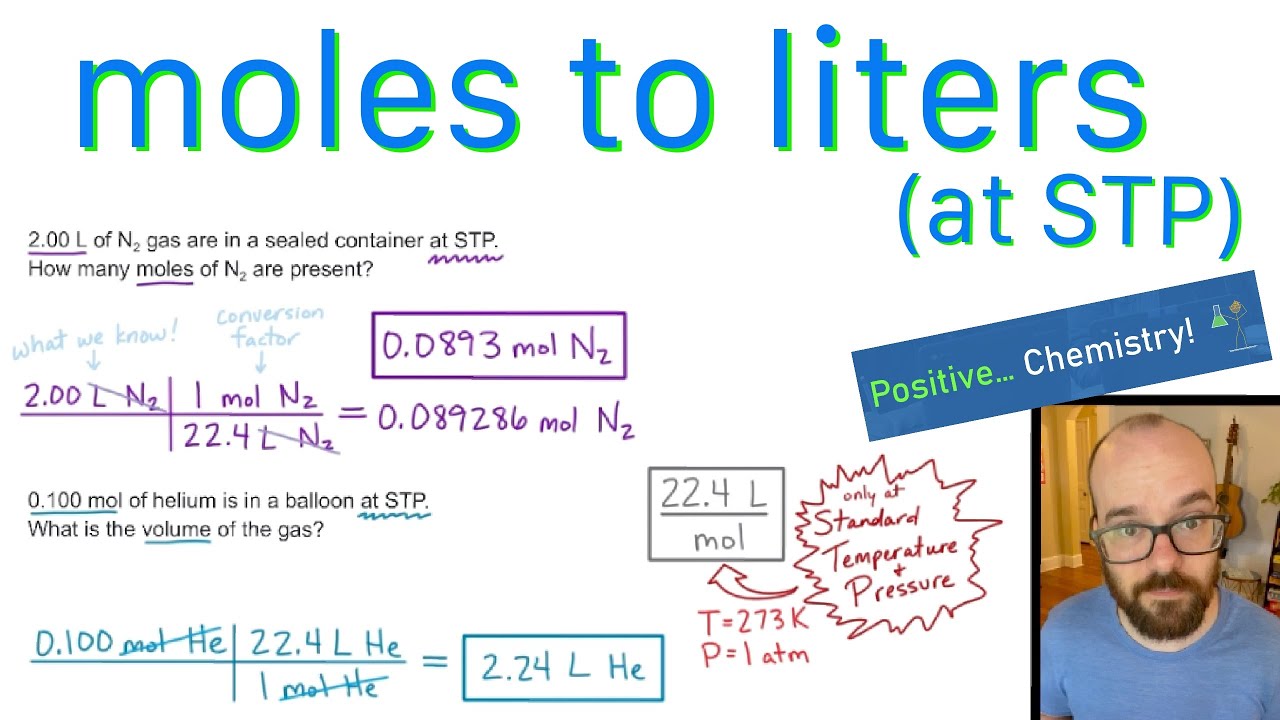
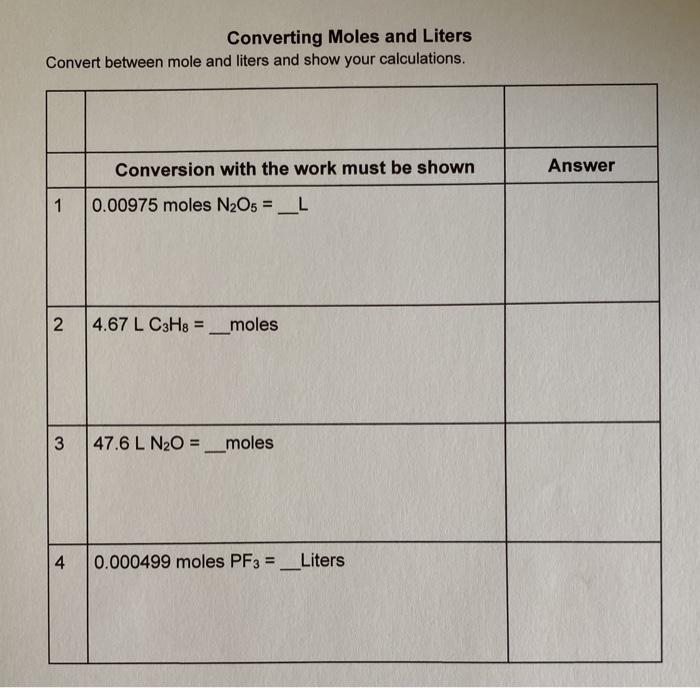
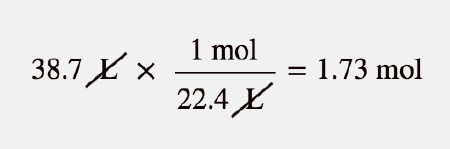SOLVED:Use molar volume to calculate each of the following at STP: a. the volume, in liters, occupied by 2.50 moles of N2 gas b. the number of moles of CO2 in 4.00

Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry - Y… | Stoichiometry chemistry, Chemistry worksheets, Chemistry
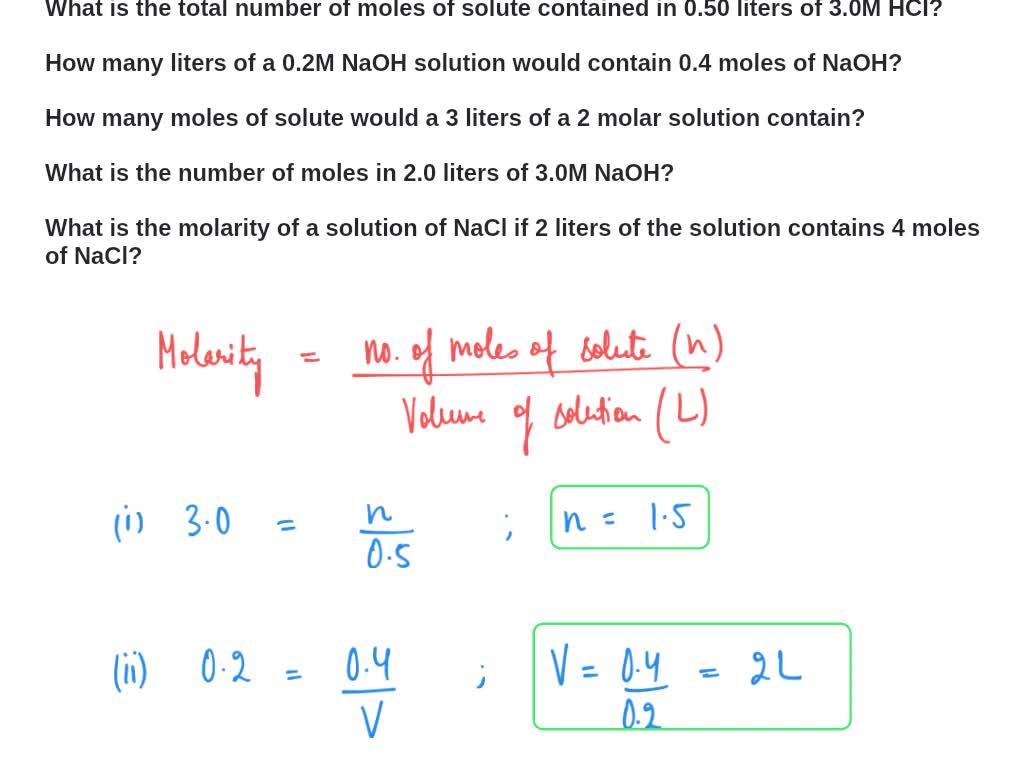
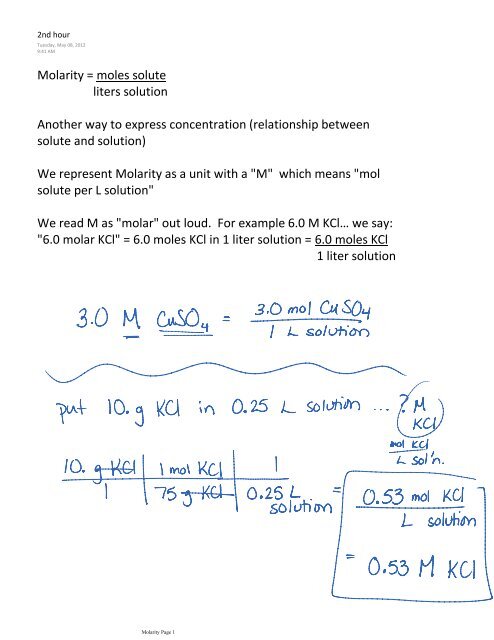
SOLVED: What is the total number of moles of solute contained in 0.50 liters of 3.0M HCl? How many liters of a 0.2M NaOH solution would contain 0.4 moles of NaOH? How

.PNG)