
Morgan Taylor Lacquer Disney Villains Collection Starter Kit Two, 3 colors + Top and Base - Nail Supply Inc

The Quest for the Ideal Base: Rational Design of a Nickel Precatalyst Enables Mild, Homogeneous C–N Cross-Coupling | Catalysis | ChemRxiv | Cambridge Open Engage

Grasping the Influence of Law on Sea Power - Scholar's Choice Edition: Kraska, James: 9781298047182: Amazon.com: Books
![Investigating the Underappreciated Hydrolytic Instability of 1,8-Diazabicyclo[5.4.0]undec-7-ene and Related Unsaturated Nitrogenous Bases | Organic Process Research & Development Investigating the Underappreciated Hydrolytic Instability of 1,8-Diazabicyclo[5.4.0]undec-7-ene and Related Unsaturated Nitrogenous Bases | Organic Process Research & Development](https://pubs.acs.org/cms/10.1021/acs.oprd.9b00187/asset/images/acs.oprd.9b00187.social.jpeg_v03)
Investigating the Underappreciated Hydrolytic Instability of 1,8-Diazabicyclo[5.4.0]undec-7-ene and Related Unsaturated Nitrogenous Bases | Organic Process Research & Development

Molecules | Free Full-Text | An Investigation of the Organoborane/Lewis Base Pairs on the Copolymerization of Propylene Oxide with Succinic Anhydride
![1,5,7‐Triazabicyclo[4.4.0]dec‐5‐ene Enhances Activity of Peroxide Intermediates in Phosphine‐Free α‐Hydroxylation of Ketones - Wang - 2021 - Angewandte Chemie - Wiley Online Library 1,5,7‐Triazabicyclo[4.4.0]dec‐5‐ene Enhances Activity of Peroxide Intermediates in Phosphine‐Free α‐Hydroxylation of Ketones - Wang - 2021 - Angewandte Chemie - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/6fc8955b-14e2-45e0-9718-323025818aed/ange202014478-fig-0001-m.jpg)
1,5,7‐Triazabicyclo[4.4.0]dec‐5‐ene Enhances Activity of Peroxide Intermediates in Phosphine‐Free α‐Hydroxylation of Ketones - Wang - 2021 - Angewandte Chemie - Wiley Online Library

Reactions of fluoronitrobenzenes with MTBD strong base in acetonitrile in the presence of water molecules - ScienceDirect

Reactions of fluoronitrobenzenes with MTBD strong base in acetonitrile in the presence of water molecules - ScienceDirect
Kinetic and equilibrium study of the deprotonation of 4-nitrophenyl[bis(ethylsulphonyl)]methane by organic bases in acetonitrile
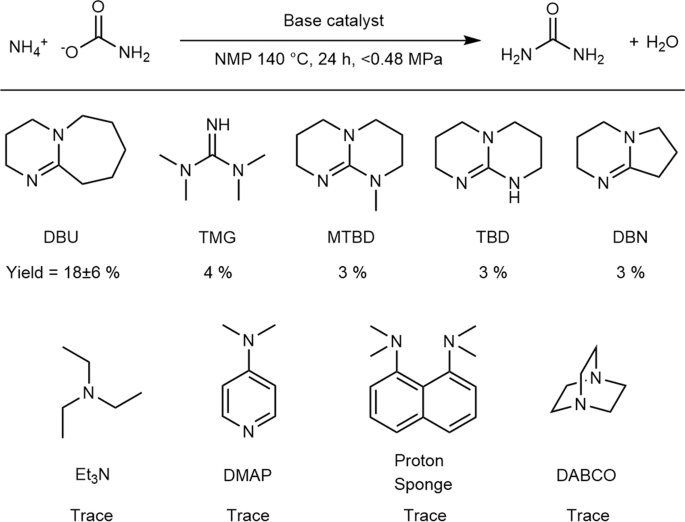
Organic bases catalyze the synthesis of urea from ammonium salts derived from recovered environmental ammonia | Scientific Reports
![Scheme 4. Hypothesized 7-Methyl-1,5,7-triazabicyclo [4.4.0]dec-5-ene... | Download Scientific Diagram Scheme 4. Hypothesized 7-Methyl-1,5,7-triazabicyclo [4.4.0]dec-5-ene... | Download Scientific Diagram](https://www.researchgate.net/publication/347740811/figure/fig3/AS:971949568176128@1608742243301/Scheme-4-Hypothesized-7-Methyl-1-5-7-triazabicyclo-440dec-5-ene-MTBD-promoted-CO2.png)




![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 95.0 %, TCI America, Quantity: 1 g | Fisher Scientific 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 95.0 %, TCI America, Quantity: 1 g | Fisher Scientific](https://assets.fishersci.com/TFS-Assets/CCG/Chemical-Structures/chemical-structure-cas-84030-20-6.jpg-650.jpg)
![Physical Properties of 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) | SpringerLink Physical Properties of 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) | SpringerLink](https://media.springernature.com/lw685/springer-static/image/art%3A10.1007%2Fs10765-019-2540-2/MediaObjects/10765_2019_2540_Fig7_HTML.png)




![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) Thermal Properties 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene (mTBD) Thermal Properties](https://thermtest.se/wp-content/uploads/2019/10/backbone-structure-dbu-tbd-mtbd.jpg)
![7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene](https://www.sigmaaldrich.com/deepweb/content/dam/sigma-aldrich/structure5/151/mfcd00043004.eps/_jcr_content/renditions/mfcd00043004-large.png)