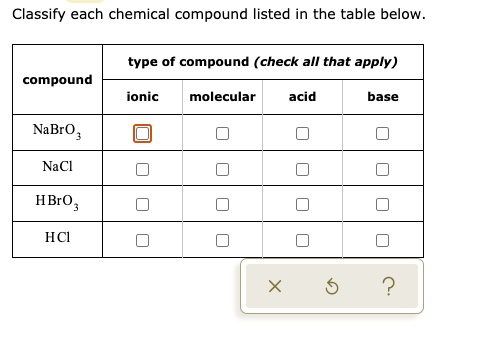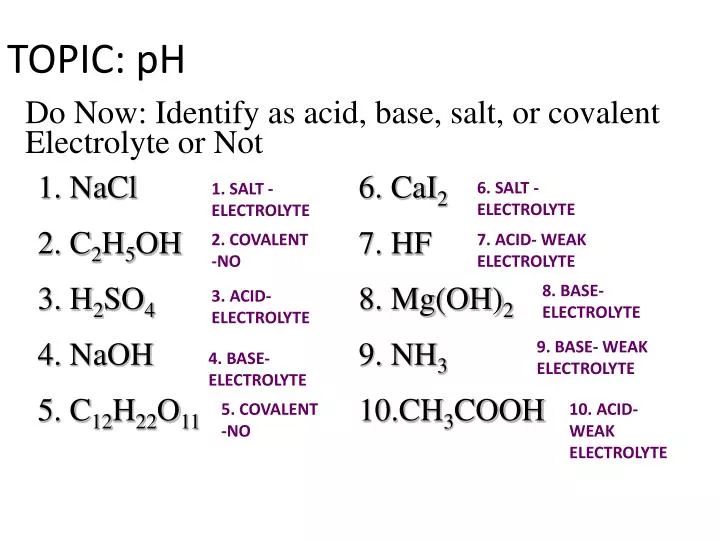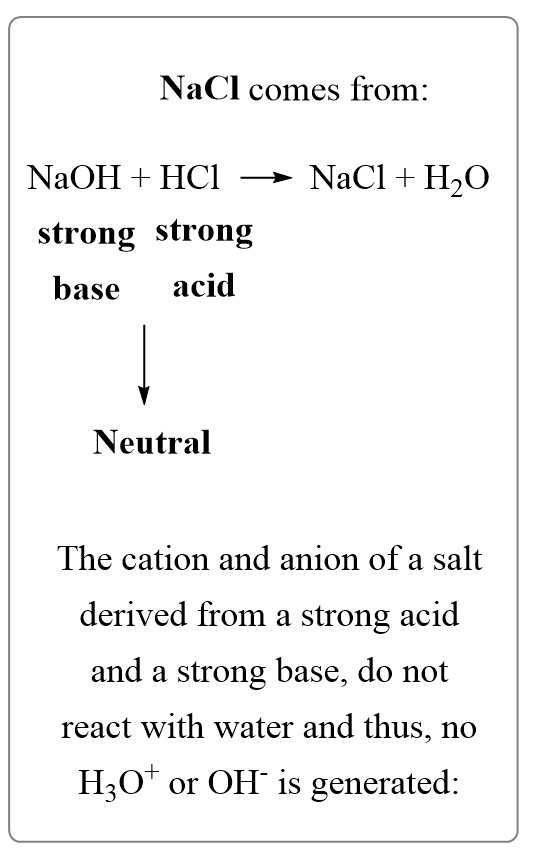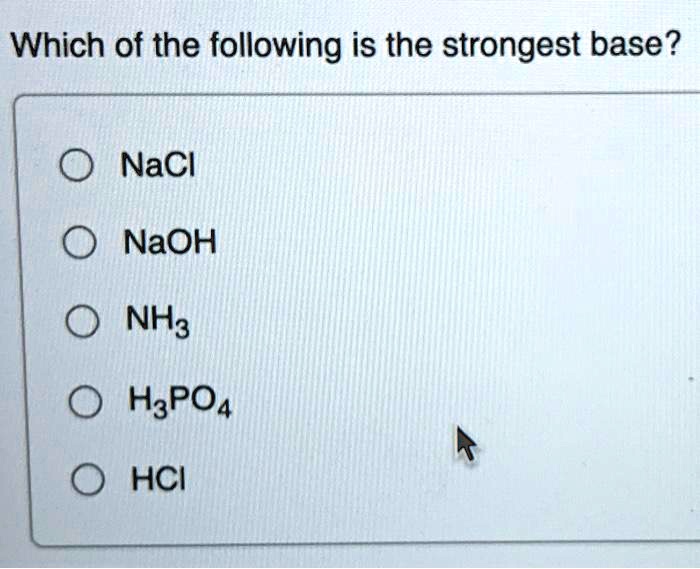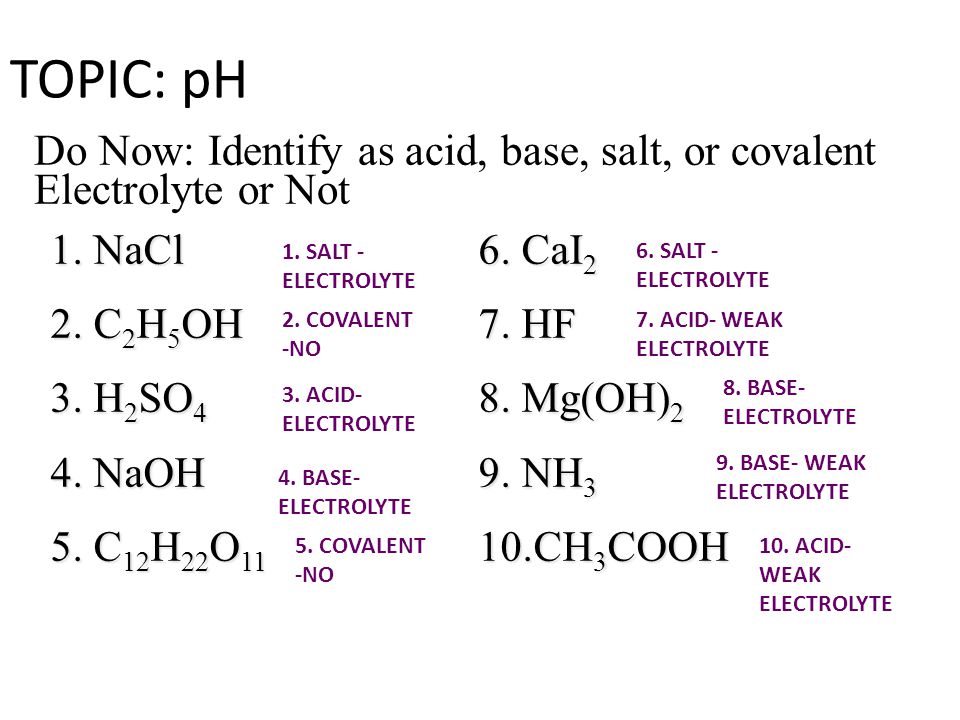
TOPIC: pH Do Now: Identify as acid, base, salt, or covalent Electrolyte or Not 1. NaCl 6. CaI2 2. C2H5OH 7. HF 3. H2SO4 8. Mg(OH)2 4. NaOH 9. NH3 5. C12H22O ppt download
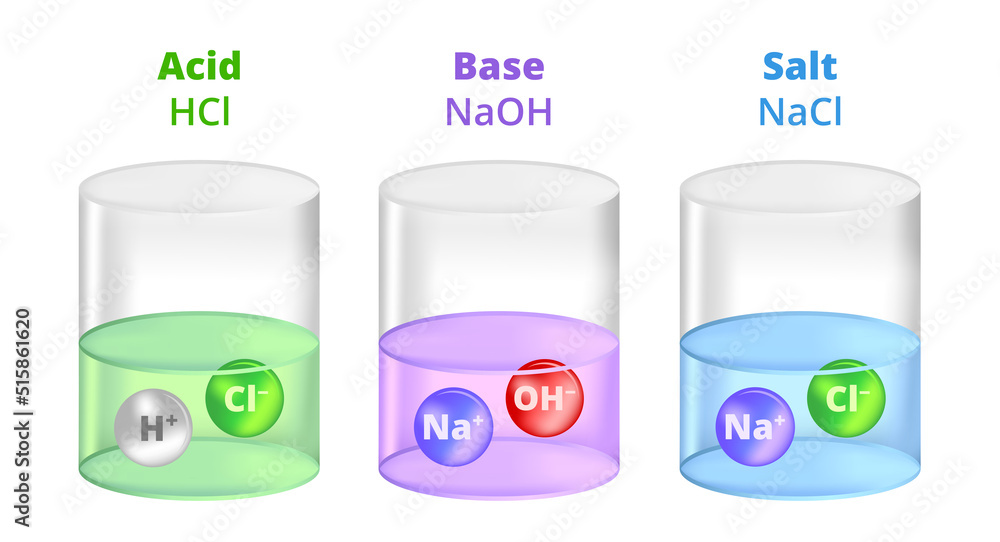
Vector illustration of electrolytic dissociation. Molecules break up into ions. Chemical containers with acid, base, and salt. HCl hydrochloric acid, NaOH sodium hydroxide, and NaCl, sodium chloride. Stock Vector | Adobe Stock

Basic properties of base material and porous aluminium alloys (NaCl... | Download Scientific Diagram

Industrial Online Acid-Base Concentration (HNO3, H2SO4, HCl, NAOH, KOH, NACL) Tester for Water Treatment (ABC-6850) - China Acid Sensor and Acid Probe

Industrial Online Acid-Base Concentration (HNO3, H2SO4, HCl, NAOH, KOH, NACL) Monitor for Water Treatment (ABC-6850) - China Acid Sensor and Acid Probe
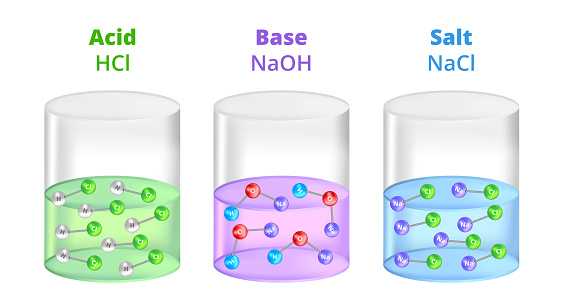
Set Of Three Chemical Containers With Acid Base And Salt With Different Ph Hcl Hydrochloric Acid Naoh Sodium Hydroxide And Nacl Sodium Chloride Stock Illustration - Download Image Now - iStock

PDF) The effect of NaCl 0.9% and NaCl 0.45% on sodium, chloride, and acid– base balance in a PICU population | Almeida Loureiro - Academia.edu
Acid–base reaction. chemical reaction neutralization. HCl hydrochloric acid, NaOH sodium hydroxide, and NaCl, sodium chloride. Vector illustration. Stock Vector | Adobe Stock