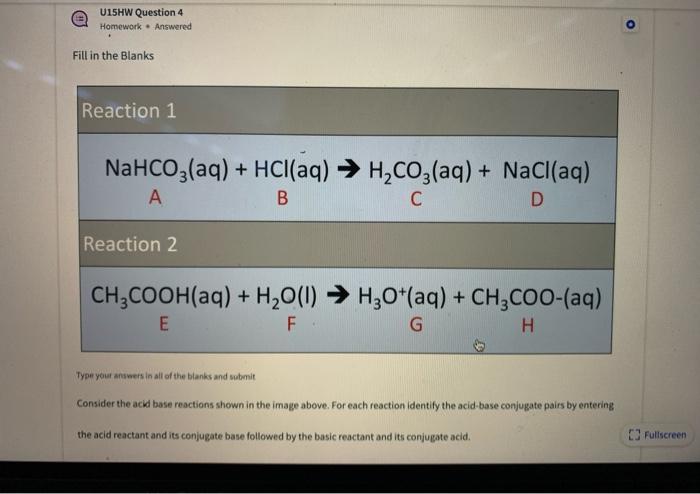
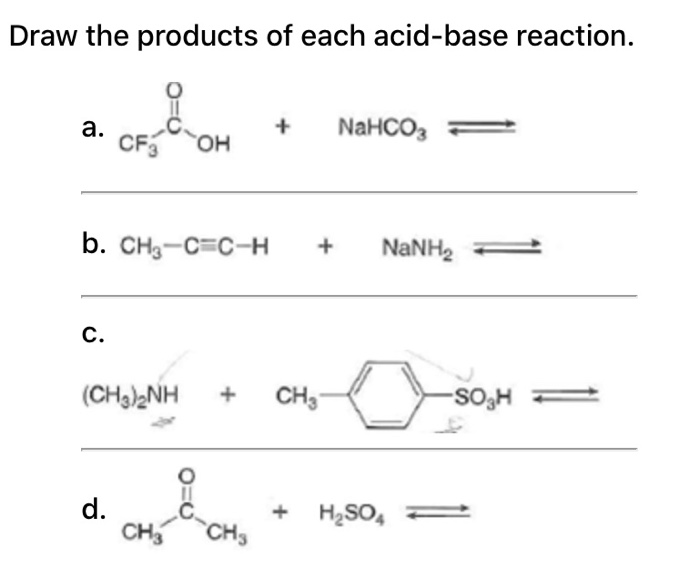
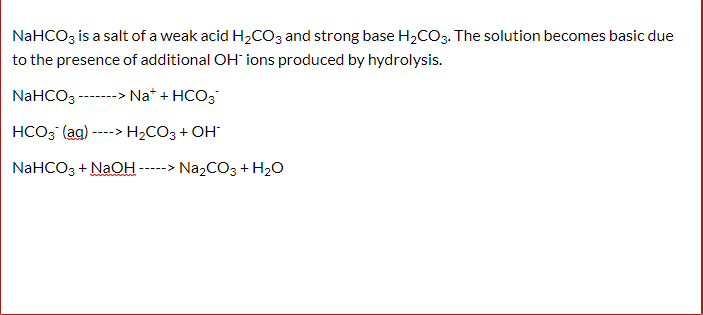
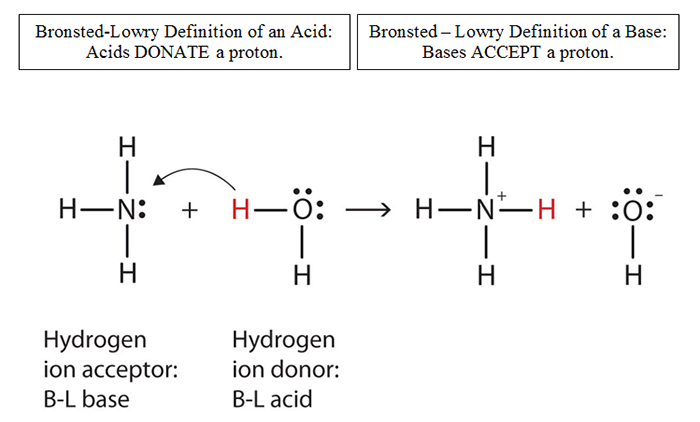
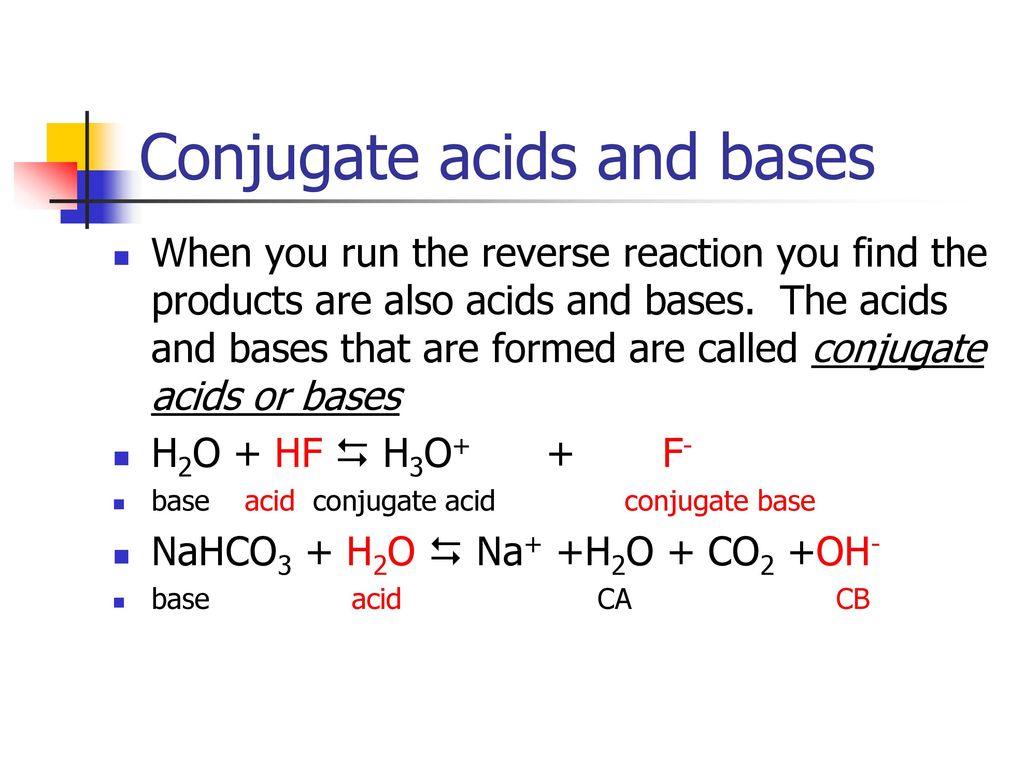
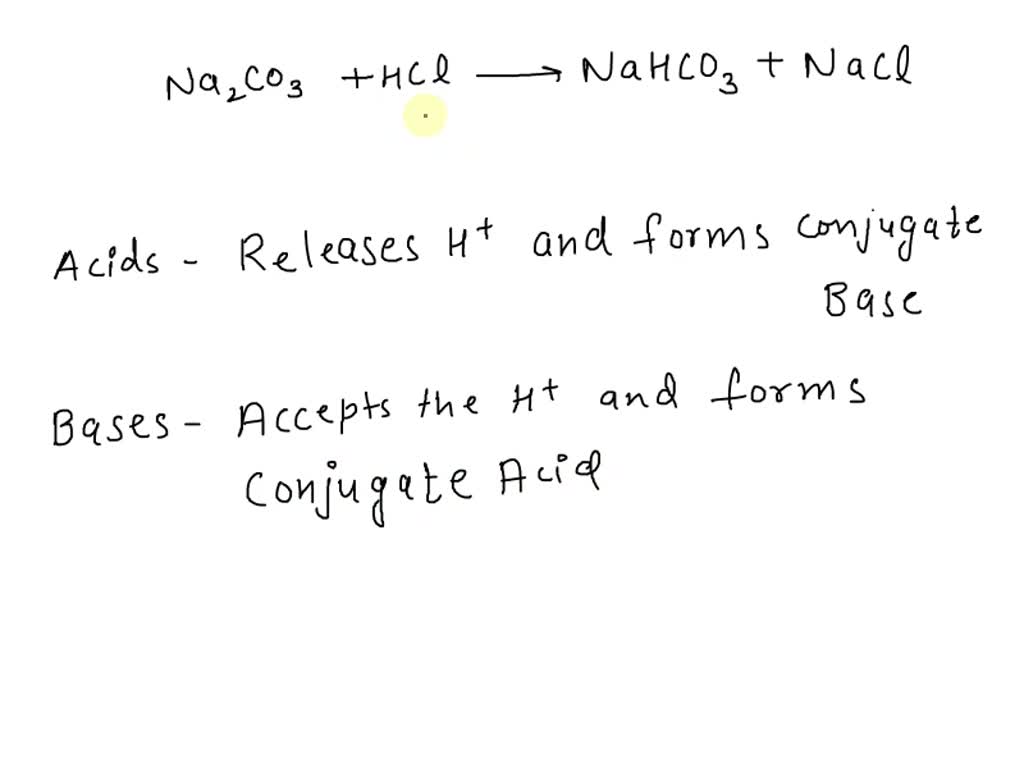
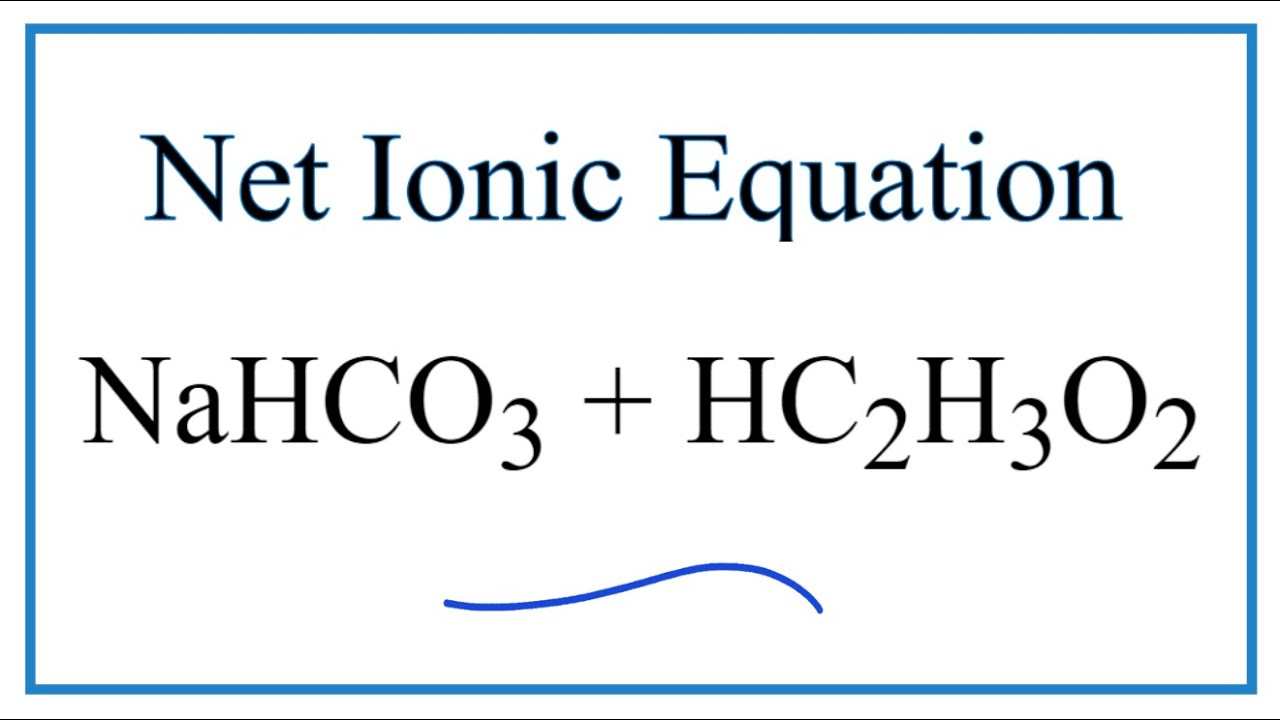Identify the acid and base which form sodium hydrogen carbonate . Write chemical equation in support of your answer. State whether its compound is acidic, basic or neutral. Also write its pH
![SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen](https://cdn.numerade.com/previews/ef6e1b45-03cf-464d-9e55-544d05108206_large.jpg)
SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen
72.Baking soda is an acidic salt or a basic salt.? (NaHCO3 is formed from a weak acid and a strong base so it should be basic but it also has one replaceable

How to Balance NaHCO3 + HCl = NaCl + CO2 + H2O (sodium bicarbonate plus hydrochloric acid) - YouTube