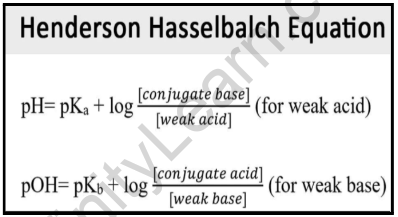
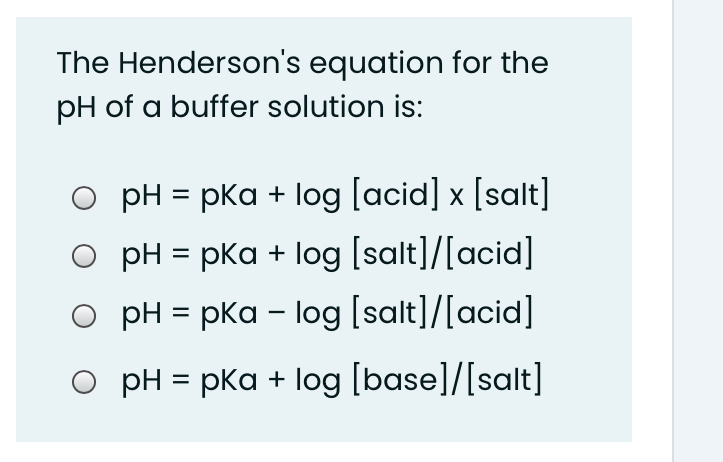
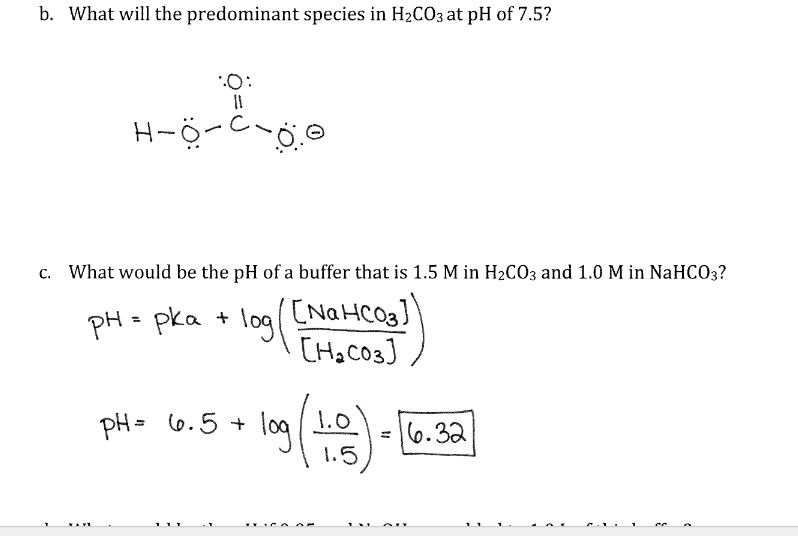
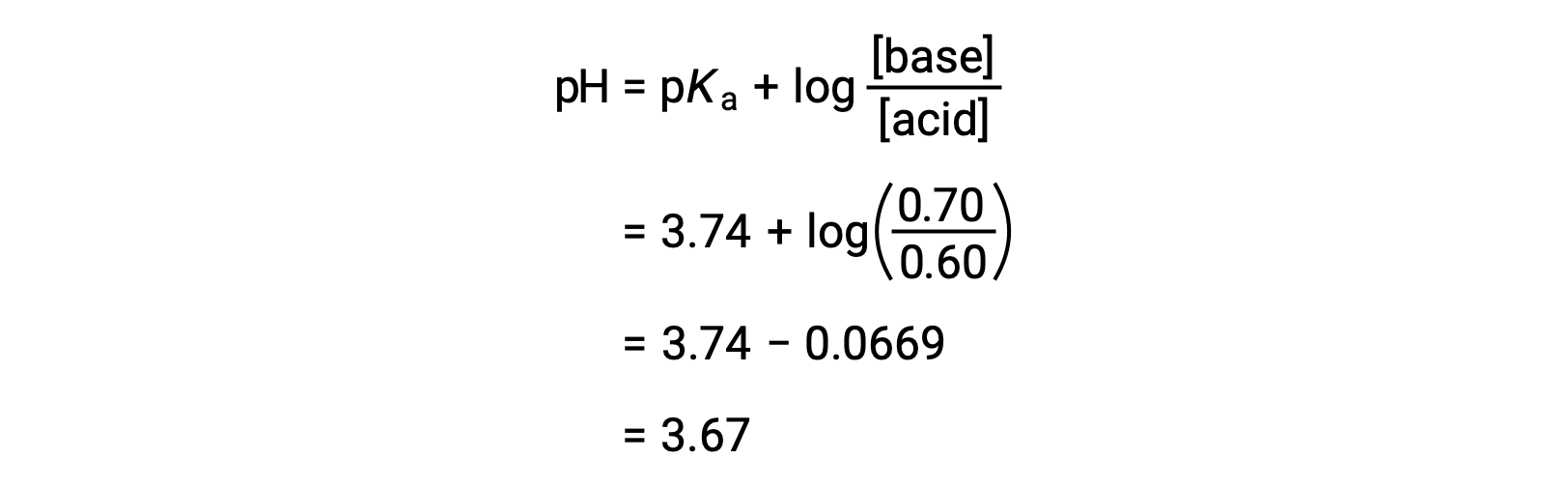Acid Base Titrations AP Chemistry Chapter 15. Titration Titrations are used to determine the amount of acid or base in a solution Titrant: the solution. - ppt download
![SOLVED: Henderson-Hasselbalch Equation (YOu must be able to derive this equation): [conjugate base] [A-] pH pKa + log OR pH = pKa + log [acid] [HA] 0. 1. What is the pH SOLVED: Henderson-Hasselbalch Equation (YOu must be able to derive this equation): [conjugate base] [A-] pH pKa + log OR pH = pKa + log [acid] [HA] 0. 1. What is the pH](https://cdn.numerade.com/ask_images/b0b5d45010ed4627b06a3a093e4b025c.jpg)
SOLVED: Henderson-Hasselbalch Equation (YOu must be able to derive this equation): [conjugate base] [A-] pH pKa + log OR pH = pKa + log [acid] [HA] 0. 1. What is the pH
![The pH of basic buffer mixtures is given by : pH=pK(a)+log((["Base"])/(["Salt"])) , whereas pH of acidic buffer mixtures is given by: pH= pK(a)+log ((["Salt"])/(["Acid"])). Addition of little acid or base although shows no The pH of basic buffer mixtures is given by : pH=pK(a)+log((["Base"])/(["Salt"])) , whereas pH of acidic buffer mixtures is given by: pH= pK(a)+log ((["Salt"])/(["Acid"])). Addition of little acid or base although shows no](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/12003548_web.png)
The pH of basic buffer mixtures is given by : pH=pK(a)+log((["Base"])/(["Salt"])) , whereas pH of acidic buffer mixtures is given by: pH= pK(a)+log ((["Salt"])/(["Acid"])). Addition of little acid or base although shows no
![Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube](https://i.ytimg.com/vi/SLPu7qlUdEA/maxresdefault.jpg)
Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube

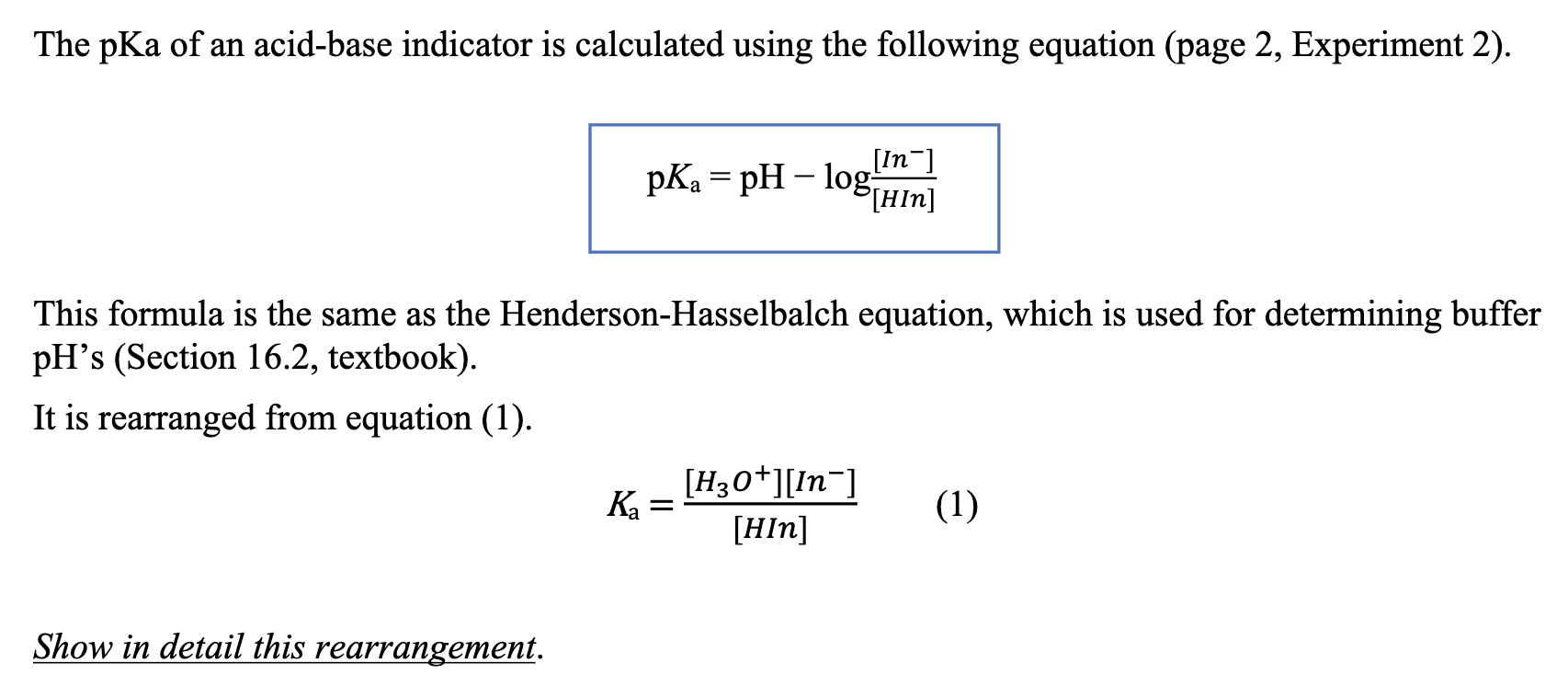
Yexel Exams - Buffer solutions are used to reduce pH fluctuations associated with introduction of small amounts of strong acids or bases. Typical buffer solutions are composed of a weak acid or

![Biochemistry | Henderson-Hasselbalch Equation Proof [pH=pKa] - YouTube Biochemistry | Henderson-Hasselbalch Equation Proof [pH=pKa] - YouTube](https://i.ytimg.com/vi/2jpB30LsT8g/maxresdefault.jpg)
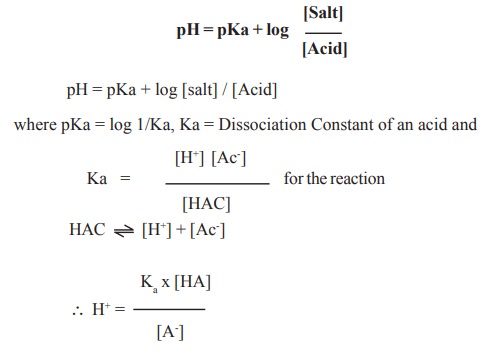
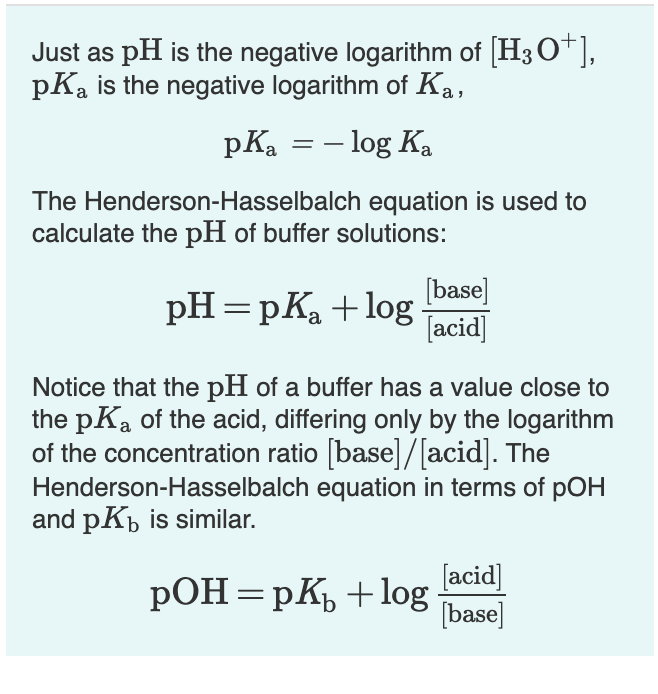
![Solved Just as pH is the negative logarithm of (H30+], pK, | Chegg.com Solved Just as pH is the negative logarithm of (H30+], pK, | Chegg.com](https://media.cheggcdn.com/media/375/3755951c-9b2e-4931-a914-33bb100113d8/php72x3Cn.png)
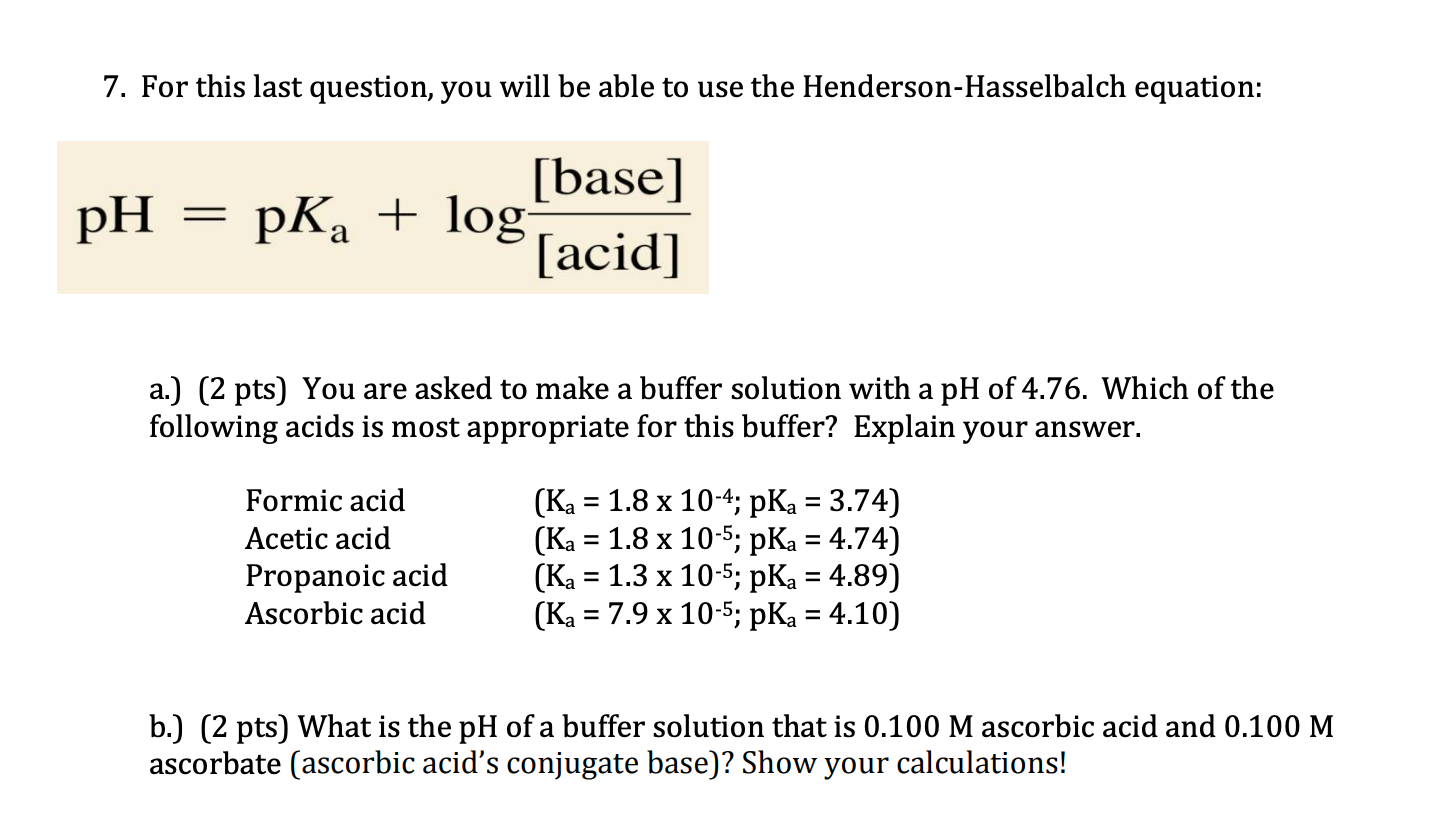

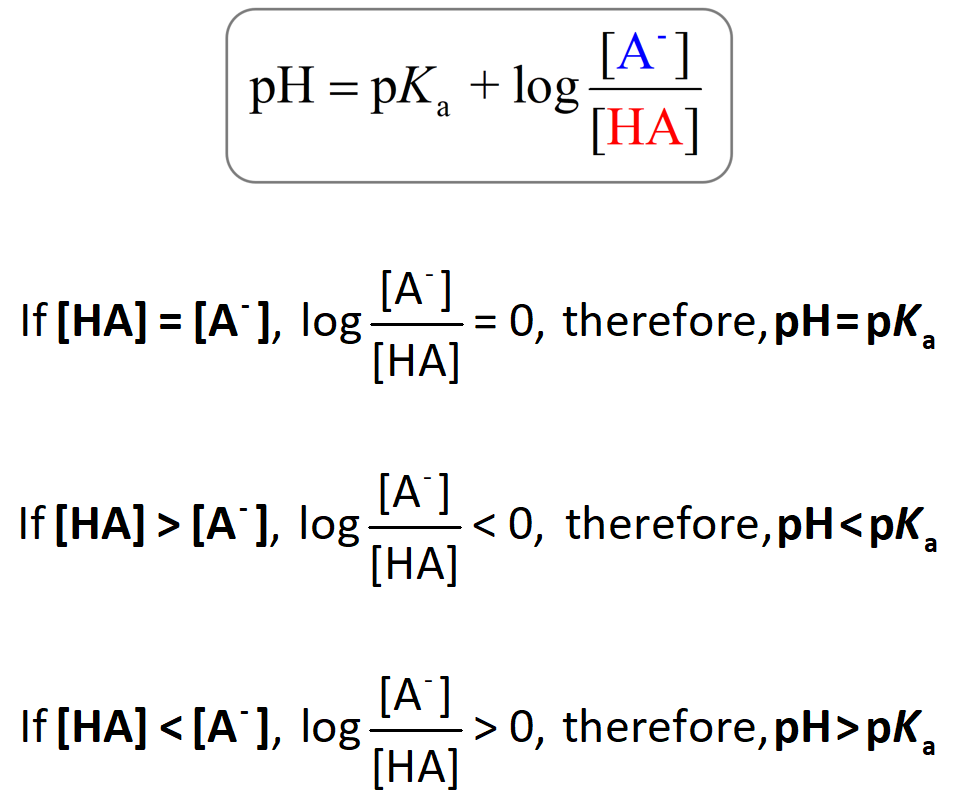

![Solved Important equations pH = pKa + log[salt]/[acid] | Chegg.com Solved Important equations pH = pKa + log[salt]/[acid] | Chegg.com](https://media.cheggcdn.com/media/14b/14b35f43-4468-42d2-a7e2-d924b3fd6eba/phpQPtBXO.png)