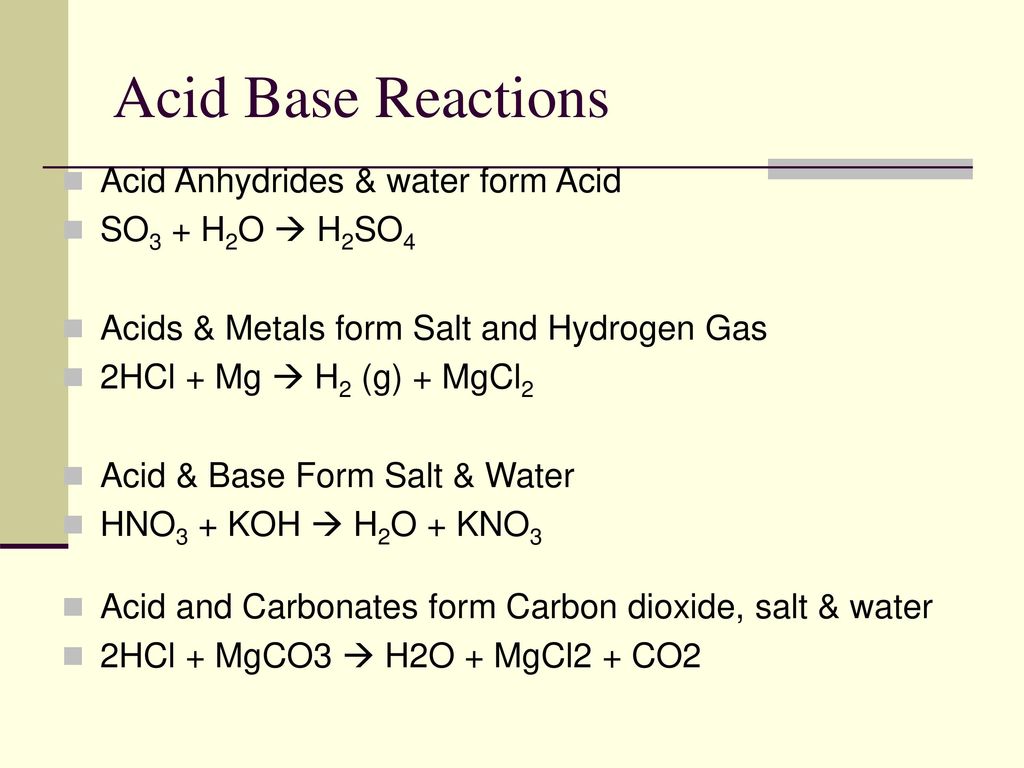
ACP - Pyruvic acid, an efficient catalyst in SO3 hydrolysis and effective clustering agent in sulfuric-acid-based new particle formation
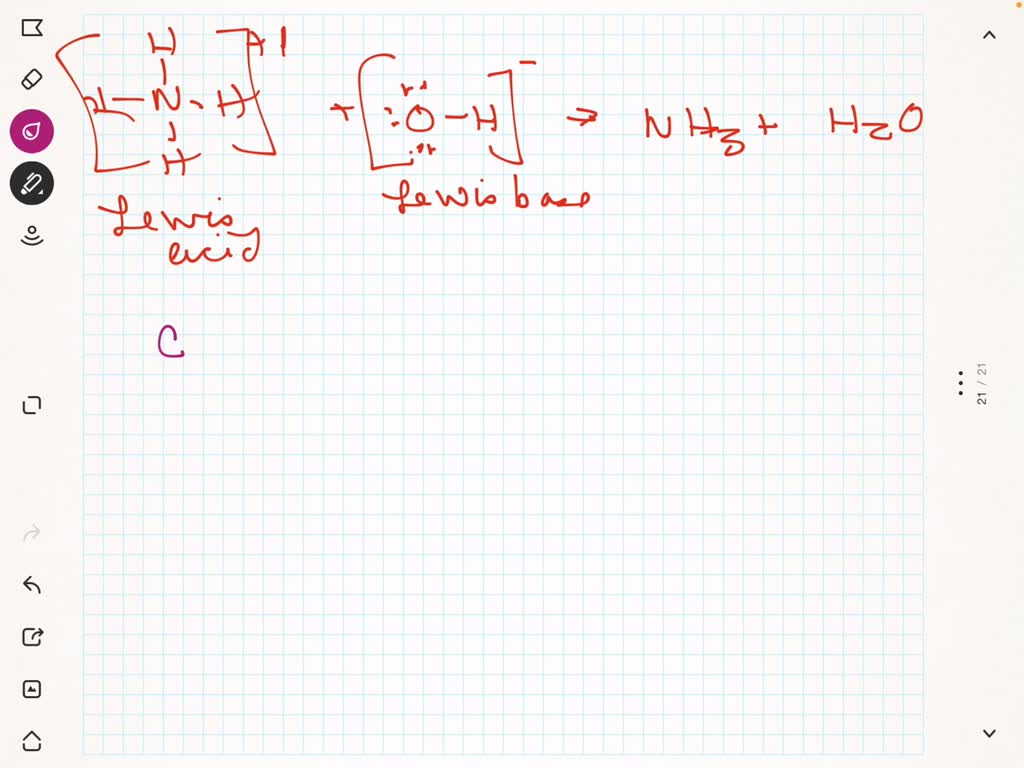
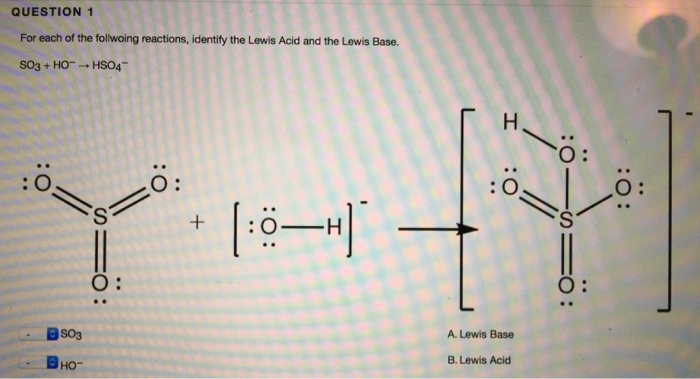
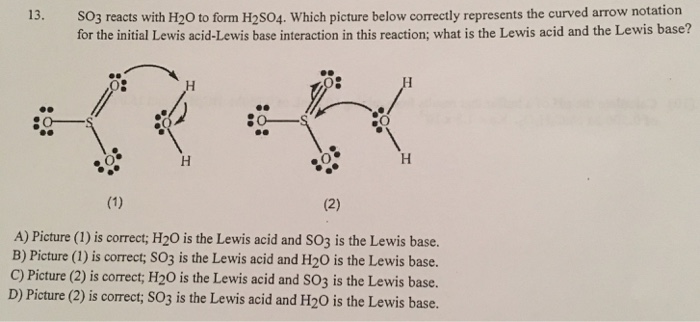
SOLVED: Answer the question below: 1: i) Identify the Lewis acids and bases in each of the following reactions: a. NH3 + BF3 → F3B NH3 b. H2O + SO3 → H2SO4
![SOLVED: 22. Based on the Lewis structures of the reactants and product of the following equation, identify the Lewis acid and the Lewis base in it: I + [ 15 6 @ [+] SOLVED: 22. Based on the Lewis structures of the reactants and product of the following equation, identify the Lewis acid and the Lewis base in it: I + [ 15 6 @ [+]](https://cdn.numerade.com/ask_images/4fe6fd50e149442ba6d53d16107fe043.jpg)
SOLVED: 22. Based on the Lewis structures of the reactants and product of the following equation, identify the Lewis acid and the Lewis base in it: I + [ 15 6 @ [+]

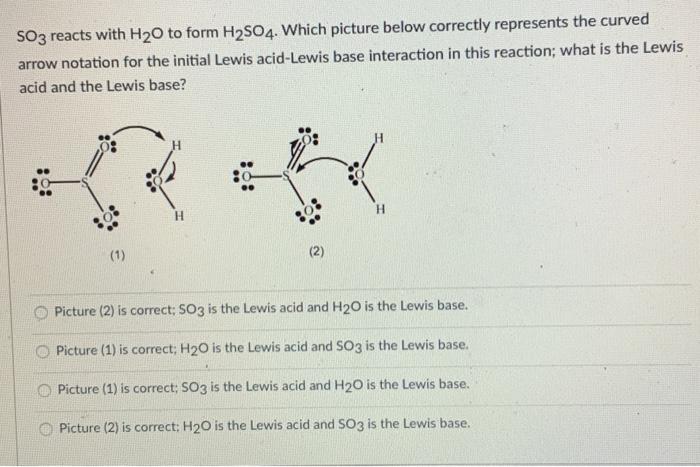
SOLVED: Q; in the reaction between H2O and SO3, SO3 acts as a Lewis acid while H2O act as a Lewis base true or false ?
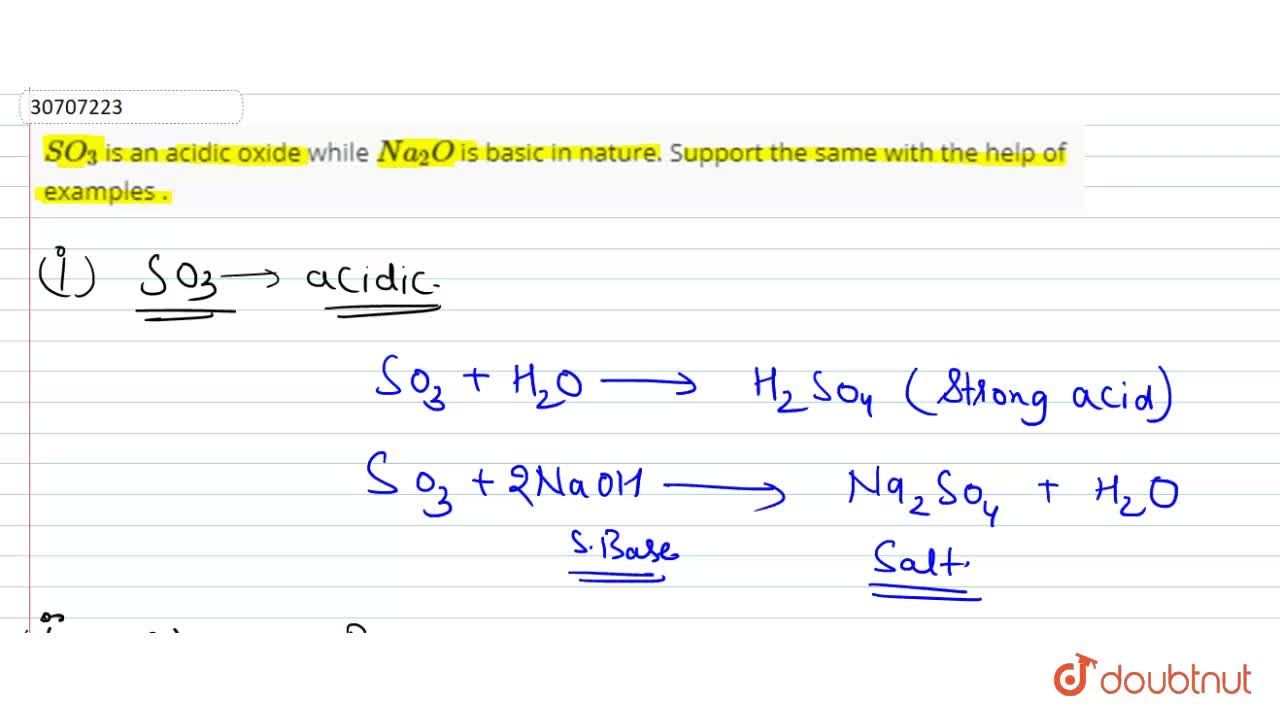
SO(3) is an acidic oxide while Na(2)O is basic in nature. Support the same with the halp of exaples .

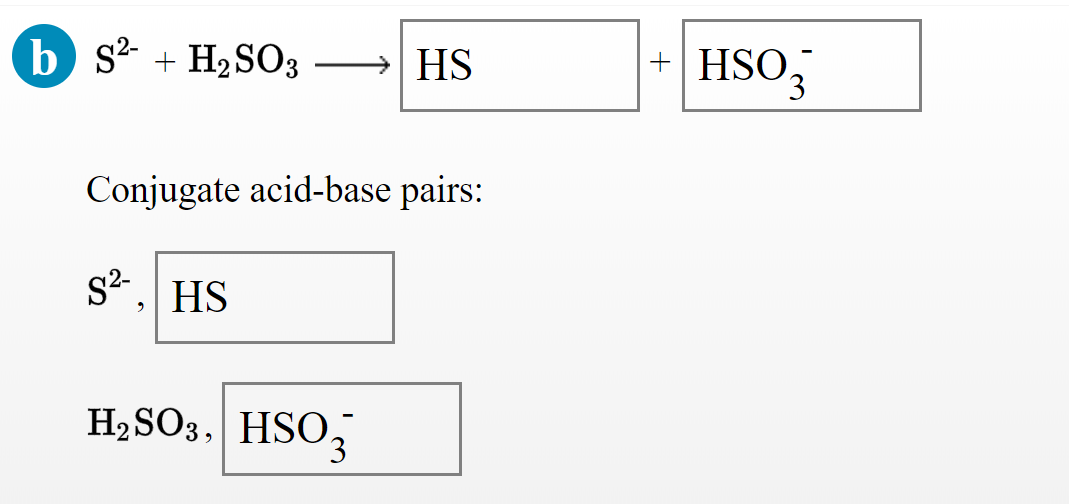
⚗️H₂O is the Lewisin thefollowing reaction.SO3(aq) + 2H₂O(1) = H₂SO3(aq) + 2OH- (aq)ABacidbase - Brainly.com