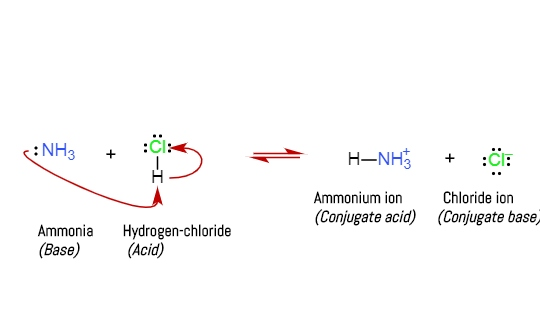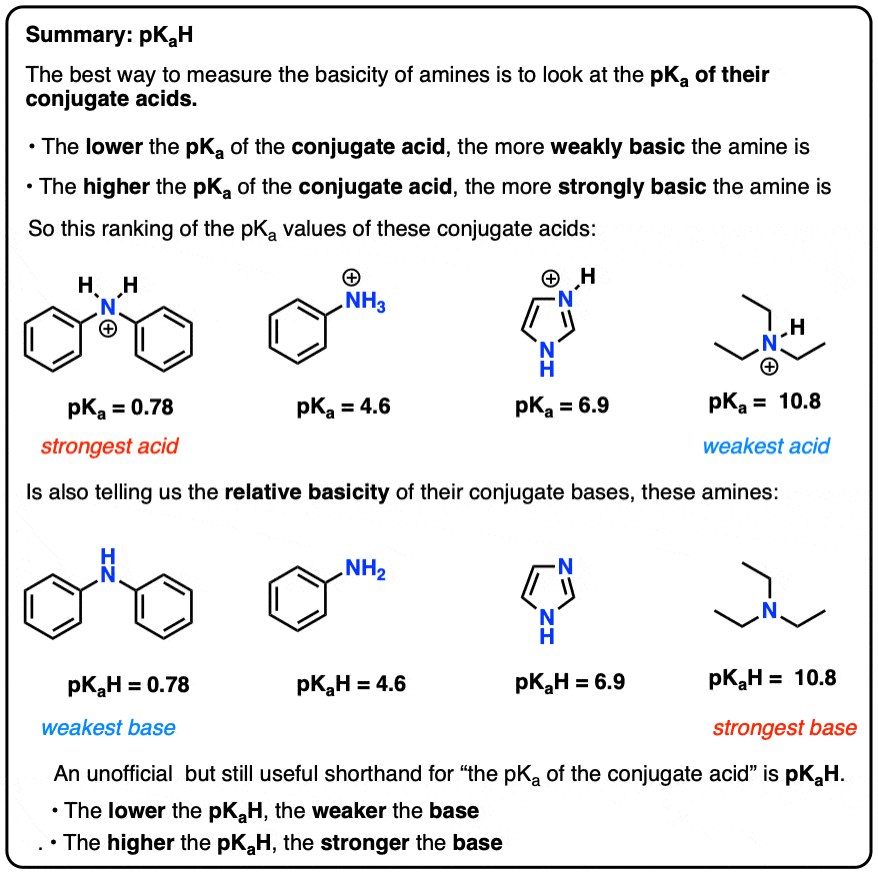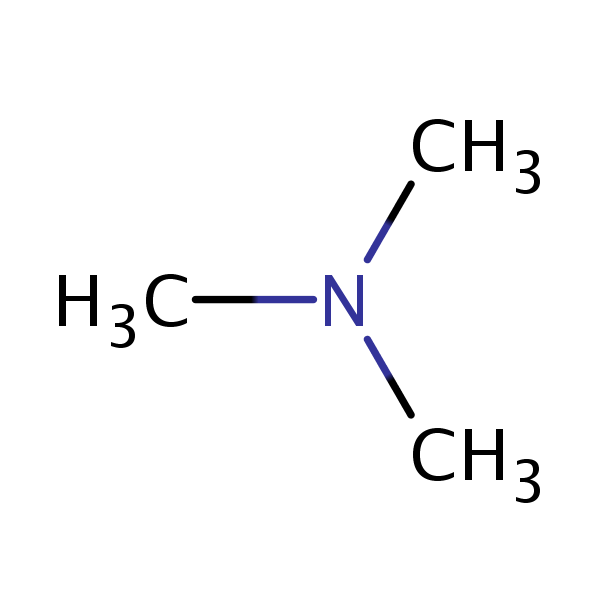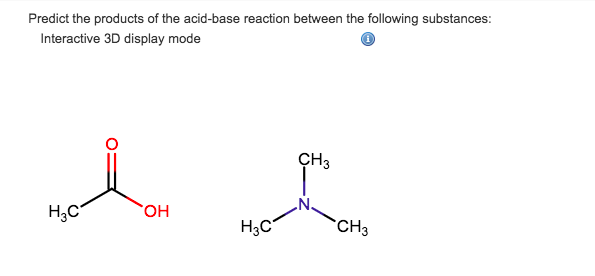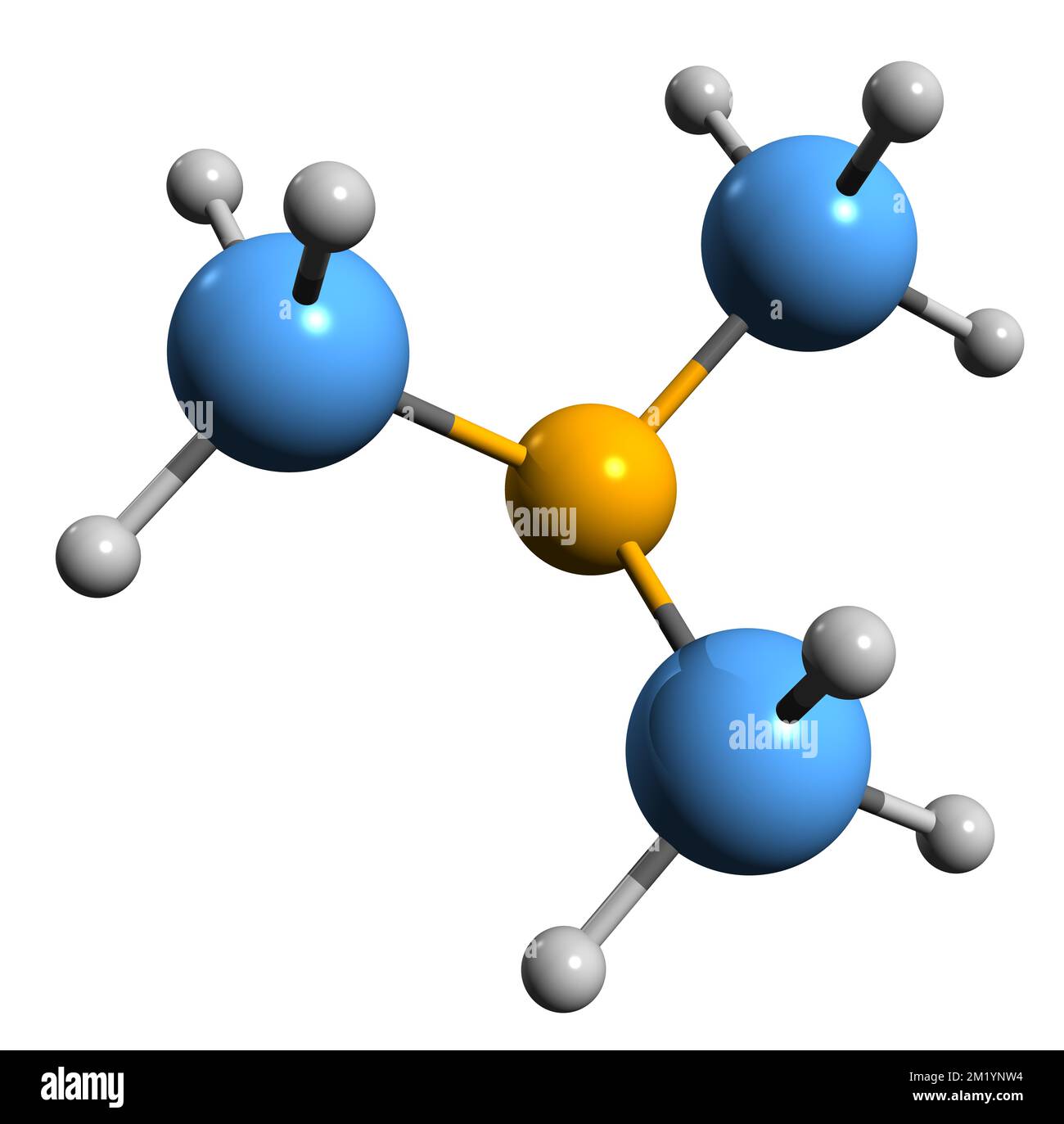
Trimethylamine is a nitrogenous base and can be protonated to trimethylammonium cation. This colorless, hygroscopic, and flammable tertiary amine has Stock Vector Image & Art - Alamy
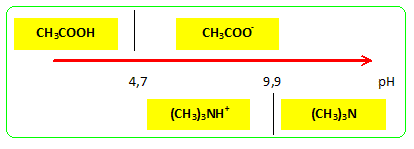
✓ Solved: Base Ionization Trimethylamine, (CH3)3N, is a gas with a fishy, ammonialike odor. An aqueous...

Pls explain: Q Trisilyl amine is a weaker base than trimethyl amine because (a) in trisilyl amine pπ-pπ bonding - Chemistry - The p-Block Elements - 12586833 | Meritnation.com
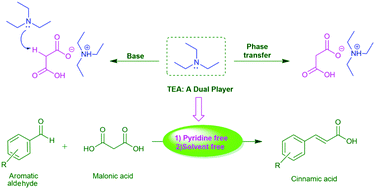
Triethylamine: a potential N-base surrogate for pyridine in Knoevenagel condensation of aromatic aldehydes and malonic acid - New Journal of Chemistry (RSC Publishing)
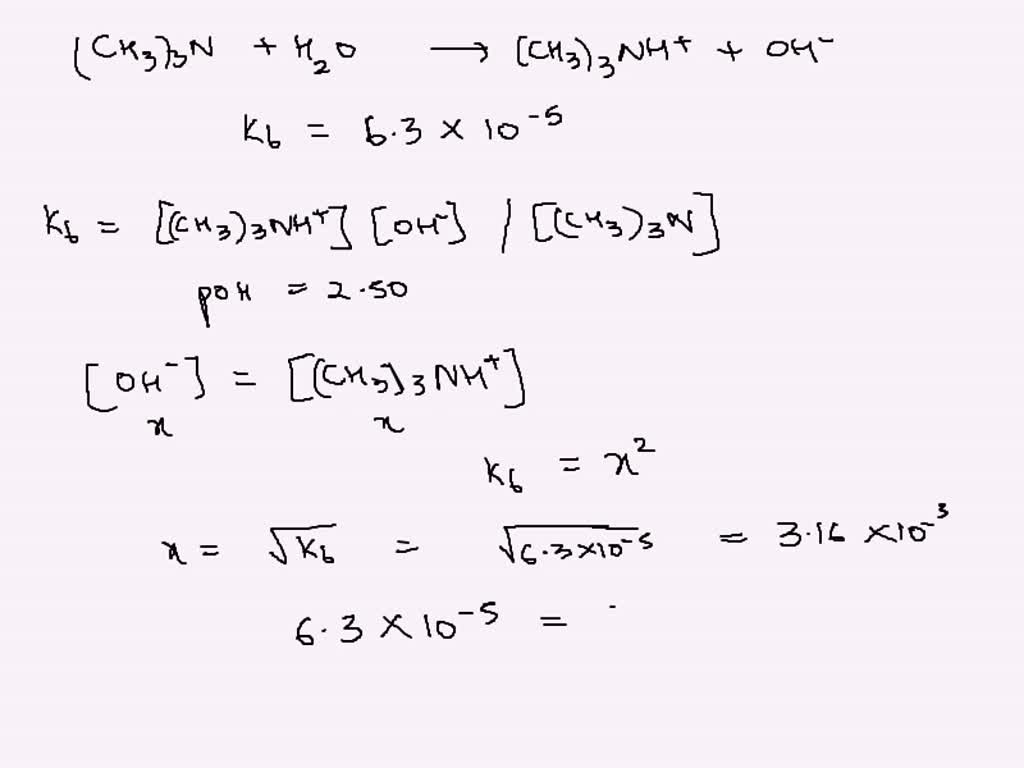
SOLVED: Trimethylamine, (CH3)3N, is a weak base (Kb = 6.3 × 10–5). What volume of this gas, measured at STP, must be dissolved in 2.5 L of solution to give that solution

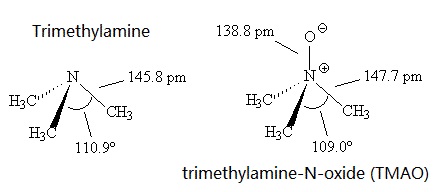
What is the Difference Between Dimethylamine and Trimethylamine | Compare the Difference Between Similar Terms

18856-86-5 | Trimethylamine-d9 Hydrochloride | N,N-Dimethylmethanamine-d9 Hydrochloride; | C₃D₉N • HCl | TRC
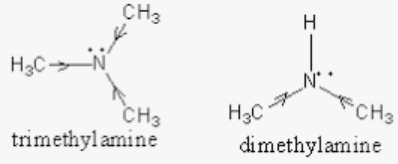
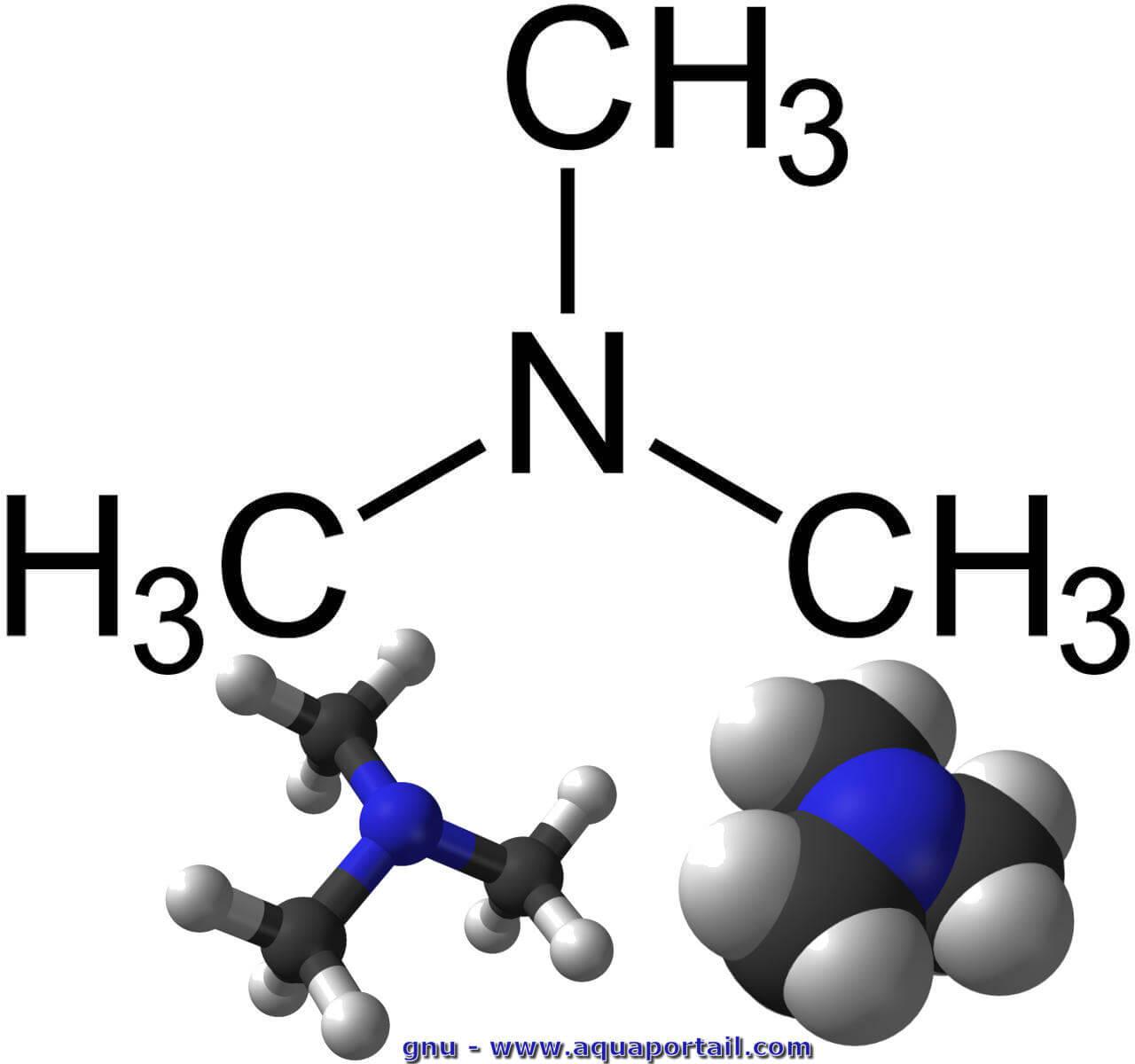
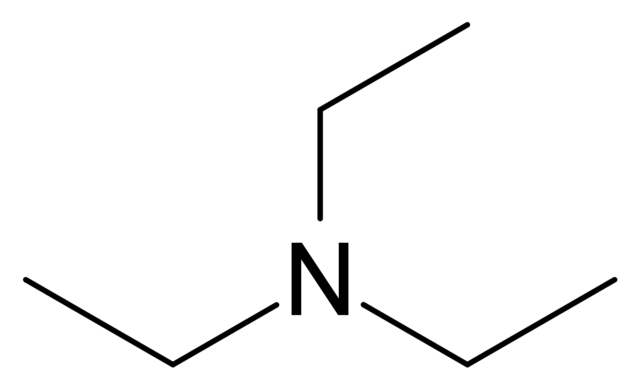
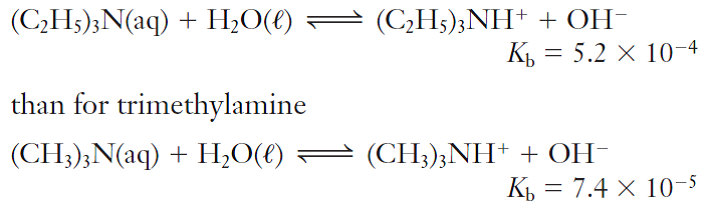
![ANSWERED] Trimethylamine, (CH3)3N, is a weak base w... - Physical Chemistry ANSWERED] Trimethylamine, (CH3)3N, is a weak base w... - Physical Chemistry](https://media.kunduz.com/media/sug-question/raw/73933115-1659733640.5428739.jpeg)